Nosy Neuroprotection: Intranasal Administration of Neuroprotective Agents to the Brain
Introduction
In everyday or emergency medical practice, intranasal (IN) administration of therapeutic agents (i.e., drug delivery via the nose) offers several advantages over oral, intravenous, and other routes of administration. Drugs can be rapidly absorbed through the large surface area of the nasal mucosa, resulting in a rapid onset of action and avoiding degradation in the gastrointestinal tract and first-pass metabolism in the liver. IN delivery is also non-invasive and essentially painless, which helps to increase patient comfort and compliance. Because of these distinct advantages, explorations into the practical applications of IN administration are becoming increasingly common and IN formulations of a wide variety of therapeutic agents now exist. Nasal administration is a logical choice for topical nasal treatments such as antihistamines and corticosteroids, and the nasal mucosa has also received attention as a viable means of systemic administration of analgesics, sedatives, hormones, cardiovascular drugs, and vaccines(1).
Importantly, the nose also serves as a direct route to the brain. In 1937, W.F. Faber placed Prussian blue dye in the nasal cavity of rabbits and later observed the dye in both the olfactory nerve and the brain, demonstrating for the first time the existence of the olfactory pathway(2). Several decades later, experimenters discovered the link between the environment and the brain by performing Faber’s experiment in reverse: dyes injected into the brain ventricles of rabbits and monkeys showed that the cerebrospinal fluid
(CSF) is drained into the lymphatic vessels and the nasal mucosa via the olfactory neurons(3,4). Thus the olfactory neurons, which function primarily as sensory cells, also provide a means for the direct transport of agents to the central nervous system (CNS).
The implications of direct nose-to-brain transport went largely unnoticed until the 1990s, when enhanced public attention to brain research compelled scientists to discover and implement effective treatment strategies in an effort to combat the upsurge of age-related neurodegenerative diseases and related neurological disorders in an increasingly elderly patient population. A consistent frustration in the treatmentof brain disease and stroke is that many drugs known to mitigate the damaging effects of such pathologies are unable to enter the brain from the systemic circulation due to the existence of a tight membranic structure called the blood-brain barrier (BBB). However, because the olfactory receptor cells are in direct contact with both the environment and the central nervous system, the olfactory pathway offers a potential means of circumventing the BBB to deliver neuroprotective agents directly to the brain. Accordingly, IN delivery of drugs targeting the CNS is now an area of great interest(5,6).
In cryonics, certain neuroprotective agents are administered to patients in an attempt to prevent (or at least slow down) ischemic damage to the brain after cardiac arrest and during the low-flow reperfusion provided by cardiopulmonary support. Cooling is, of course, our primary line of defense against such damage because of its striking effectiveness in reducing metabolic demand. However, rapid field cooling still presents considerable logistical and clinical challenges and preferential brain cooling is (at least until the patient arrives in the operating room) yet to be accomplished. Therefore, quick and direct protection of the brain is especially important in the moments following pronouncement and during the initial stages of cooling, while the patient is still relatively warm.
Currently, all medications delivered to cryonics patients are introduced to the systemic circulation either via the intravenous (IV) or intraosseous (IO) routes, requiring skilled personnel who have been trained in IV and/or IO technique. Due to the blood-brain barrier and first-pass metabolism, relatively large volumes ofneuroprotective agents must be given in order for an effective dose to enter the brain via the systemic circulation. In fact, some of the most promising neuroprotective drugs are currently not available at all for treatment of cryonics patients because of poor BBB permeability. Additionally, IN administration of cardiovascular drugs has been a growing topic of investigation(7). IN propanalol provides immediate β-blockade when taken before exercise by patients with angina. IN administration of propanalol exhibits a pharmacokinetic profile similar to IV administration and 10 times greater bioavailibility than oral propanalol (because oral propanalol undergoes considerable first-pass metabolism in the liver). In cryonics, patients may also benefit from IN administration of vasopressors (i.e., medications for the maintenance of blood pressure) during stabilization. IN epinephrine has been shown to reach peak plasma concentrations in only 15 seconds(8), and to improve coronary perfusion pressure in canine models of cardiac arrest and CPR(9). Indeed, IN administration of epinephrine is a rapidly obtainable and feasible route of administration during any cardiac emergency.
The Nose
The nasal cavity is split into two symmetrical halves by the nasal septum (comprised of cartilidge and bone) with each side opening at the face via the nostrils and connecting with the mouth at the nasopharynx. The three main regions of the nasal cavity are the nasal vestibule, the respiratory region, and the olfactory region, reflecting the primary nasal functions of vocalization, respiration, and olfaction(10). The main nasal airway passages are narrow (only 1-3 mm wide), causing inhaled air to come into contact with the nasal mucosa, where particles such as dust and bacteria are filtered by mucociliary clearance. Simultaneously, the air is warmed by the highly-vascularized nasal epithelium and humidified by fluid secreted by the mucosa(10,11). The epithelial tissue within the nasal cavity has an extensive blood supply which drains blood from the nasal mucosa directly to the systemic circulation(12), thus providing a potential conduit for drug delivery which circumvents first-pass metabolism.
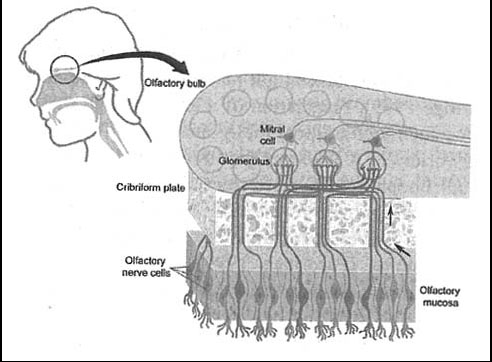
Figure 1. The olfactory bulb, olfactory mucosa, and olfactory nerve cells in humans. Modified picture from the Nobel Prize official homepage (NobelPrize.org).
The Respiratory and Olfactory Epithelium
The respiratory epithelium, making up the middle and posterior thirds of the nasal cavity, can be described as a pseudo-stratified ciliated columnar epithelium consisting of four main cell types: ciliated and non-ciliated columnar cells, goblet cells, and basal cells. The epithelial cell layer is covered with mucus, which is produced by the goblet cells and cleared by the beating of the cilia. This clearance mechanism protects the respiratory tract and lungs from bacteria and other exogenous compounds. In humans, the
respiratory mucosa covers most of the total nasal surface area and is the major site for drug absorption into the systemic circulation.
The olfactory epithelium is located at the top of the nose between the superior turbinate and the roof of the nasal cavity, just beneath the cribiform plate of the ethmoid bone (Fig. 1). In humans, it covers only 10-20 cm2, or about 8% of the total nasal surface area, and is composed of three main cell types: olfactory receptor neurons, supporting cells, and basal cells(13,5). The olfactory epithelium is more than twice the depth of the respiratory epithelium, with the olfactory nerve cell bodies typically located in the middle and deeper regions of the epithelium while nuclei of the supporting cells are organized in a single layer closer to the mucosal surface. Tight junctions exist between the supporting cells and between the supporting cells and olfactory
nerve cells(14).
The olfactory receptor neurons are bipolar neurons that connect the olfactory bulb of the brain with the nasal cavity. The single dendrites of the olfactory cells terminate in olfactory knobs that project above the epithelial surface and exhibit 10-25 immobile cilia which contain receptors for binding odorant molecules. The axons of the olfactory receptor neurons extend from the cell bodies and pass through the lamina propria and cribiform plate as grouped bundles (Cranial nerve I) surrounded by Schwann’s cells and perineural cells(3.) After entering the olfactory bulb, the axons synapse with juxtaglomerular neurons and with tufted and mitral cells in the glomeruli(15). Surrounding brain structures include those of the olfactory area (lateral olfactory tract, olfactory tubercle, and olfactory nucleus) and limbic system (amygdala, pre-pyriform cortex, enthorhinal cortex, hippocampus, thalamus, and hypothalamus)(8).
Nasal Transport Routes
After nasal delivery drugs first reach the respiratory epithelium, where compounds can be absorbed into the systemic circulation utilizing the same pathways as any other epithelia in the body: transcellular and paracellular passive absorption, carrier-mediated transport, and absorption through trancytosis. Although absorption across the respiratory epithelium is the major transport pathway for nasally-administered drugs and may represent a potentially timesaving route for the administration of certain systemic drugs delivered in cryonics medication protocols (e.g., epinephrine or vasopressin), the author considers the problem of BBB-mediated exclusion of brain-therapeutic agents to be of greater immediate concern. Accordingly, the remainder of this article will deal primarily with the transport of drugs to the CNS by way of the olfactory epithelium.
When a nasal drug formulation is delivered deep and high enough into the nasal cavity, the olfactory mucosa may be reached and drug transport into the brain and/or CSF via the olfactory receptor neurons may occur. The olfactory pathways may be broadly classified into two possible routes: the olfactory nerve pathway (axonal transport) and the olfactory epithelial pathway(13).
Axonal transport is considered a slow route whereby an agent enters the olfactory neuron via endocytotic or pinocytotic mechanisms and travels to the olfactory bulb by utilizing the same anterograde axonal transport mechanisms the cell uses to transport endogenous substances to the brain. Depending on the substance administered, axonal transport rates range from 20-400 mm/day to a slower 0.1-4 mm/day(16). The epithelial pathway is a significantly faster route for direct nose-to-brain transfer, whereby compounds pass paracellularly across the olfactory epithelium into the perineural space, which is continuous with the subarachnoid space and in direct contact with the CSF. Then the molecules can diffuse into the brain tissue or will be cleared by the CSF flow into the lymphatic vessels and susequently into the systemic circulation.
Factors Affecting Nasal Drug Delivery to the Brain
The size of the molecule is the major determinant in whether a substance will be absorbed across the nasal respiratory epithelium and/or transported along the olfactory pathway. Fisher et al. demonstrated an almost linear relationship between the log (molecular weight) and the log (% drug absorbed) of water-soluble compounds (190-70,000 Da)(17,18). In general, molecules weighing more than 1000 Da are absorbed far less efficiently than smaller molecules(19). However, the bioavailability of larger molecules may be increased with the use of permeation enhancers.
Other factors affecting delivery to the brain include the degree of dissocation (determined by the pKa of a substance and the pH in the surrounding area)(20), and lipophilicity (higher lipophilicity results in better transport)(21). Once a drug is in the brain, it can be further influenced by BBB efflux transporter systems like P-glycoprotein (P-gp)(22). Graff and Pollack (2003), however, found that uptake into the brain was enhanced when drugs were administered in combination with the P-gp efflux inhibitor, rifampin.
Nose-to-Brain Research
Researching nose-to-brain transfer of drugs in humans must, for obvious reasons, either employ indirect visualization of drug transfer (e.g., effects on event-related-potentials), measurement of drug concentrations in the CSF during surgery, or simple monitoring of CNS effects. Such studies have clearly indicated that drugs can be delivered to the brain in this manner, but they give no clear-cut evidence regarding the role of transfer. Because of this limitation, studies of the olfactory pathway as a conduit for transmission of drugs to the CNS have mostly made use of animals having substantially different ratios of olfactory-to-respiratory epithelium than humans. However, the mechanisms of transfer remain the same and are worthy of thorough investigation. To date, more than 50 drugs and drug-related compounds have been reported to reach the CNS after nasal administration in different species.
A growing number of recent reports have demonstrated the effectiveness of intranasal administration of neuroprotective agents in decreasing ischemic brain injury. For example, Ying et al. (2007) recently reported that intranasal administration of NAD+ profoundly decreased brain injury in a rat model of transient focal ischemia(23). Similarly, Wei et al. (2007) showed that intranasal administration of the PARG inhibitor gallotannin decreased ishemic brain injury in rats(24). Such agents are believed to provide neuroprotection by diminishing or abolishing activation of poly(ADP-ribose) polymerase-1 (PARP-1), which plays a significant role in ishemic brain damage. NAD+ was observed to reduce infarct formation by up to 86% even when administered at 2 hours after ischemic onset. Because PARP activation appears to be a downstream ischemic event, it may be worthwhile to also investigate the ability of IN administration of agents such as antiporters or NMDA receptor blockers to provide neuroprotection against the more upstream events of global ischemia such as membrane depolarization and excitotoxicity.
Applicability to Cryonics
IN administration of neuroprotective agents would appear to be a possible way to circumvent many of the problems encountered when attempting to administer neuroprotective agents during a typical cryonics case. No special training is required to administer drugs to the nasal cavity – IN administration is easy enough even for relatively unskilled stabilization team members and does not require sterile technique. Indeed, using a simple instrument such as the MAD® Nasal drug delivery device (Fig. 2) provides the possibility of self-administration of neuroprotective and cardiovascular agents in emergency situations (e.g., at the first sign of cardiac arrest). Consequently, time-sensitive ischemia medications can be administered and distributed throughout the brain more quickly and, importantly, can provide protection to the brain before cooling is sufficient to prevent major ischemic damage.
There is also evidence indicating that many agents active in the CNS are more effective when given nasally than when given by other routes. This suggests that smaller doses may be used when bypassing the BBB in this manner, allowing for the possibility of rapid administration of neuroprotective agents that work in low dosages immediately after cardiac arrest.
Limitations on the use of intranasal delivery as a means to bypass the BBB still exist, including limitation of the concentrations achievable in different regions of the brain, which will vary with each agent. Indeed, data regarding IN transport rates and bioavailability of specific drugs are still scarce. However, the advantages of IN administration appear considerable and worth further investigation to determine the extent to which they may benefit cryonics.
Figure 2. MAD nasal drug delivery device by Wolfe Tory Medical, Inc. (http://www.wolfetory.com/nasal.html)
This article originally published in Cryonics Magazine, Volume 28:3, 18-20.
——————————————————————————
1. Costantino H.R., Lisbeth I., Brandt G., Johnson P.H., Quay S.C. (2007) Intranasal delivery: Physicochemical
and therapeutic aspects. International Journal of Pharmaceutics 337: 1-24.
2. Faber W.F.(1937) The nasal mucosa and the subarachnoid space. American Journal of Anatomy 62: 121-148.
3. Jackson R.T., Tigges J., Arnold W. (1979) Subarachnoid space of the CNS, nasal mucosa, and lymphatic
system. Archives of Otolaryngology 105: 180-184.
4. Yoffey J.M. (1958) Passage of fluid and other substances through the nasal mucosa. Journal of Laryngology and Otology 72: 377-383.
5. Illum L. (2004) Is nose-to-brain transport of drugs in man a reality? Journal of Pharmacy and Pharmacology 56: 3-17.
6. Vyas T.K., Salphati I., Benet L.Z. (2005) Intranasal drug delivery for brain targeting. Current Drug Delivery
2: 165-175.
7. Landau A.J., Eberhardt R.T., Frishman W.H. (1994) Intranasal delivery of cardiovascular agents: An innovative approach to cardiovascular pharmacotherapy. American Heart Journal 127: 1594-1599.
8. Yamada T. (2004) The potential of the nasal mucosa route for emergency drug administration via a high-pressure needleless injection system. Anesthesia Progress 51(2): 6-61.
9. Bleske B.E., Warren E.W., Rice T.L., Shea M.J., Amidon G., Knight P. (1992) Comparison of intravenous and intranasal administration of epinephrine during CPR in a canine model. Annals of Emergency Medicine 21(9): 1125-1130.
10. Chien Y.W., Su K.S.E., Chang S.F. (1989) Nasal systemic drug delivery. Drugs and the pharmaceutical
sciences. New York, Marcel Dekker, Inc.
11. Proctor F. (1973) Clearance of inhaled particles from the human nose. Archives of Internal Medicine
131: 132-139.
12. Mygind N., Pedersen M., Nielsen M.H. (1982) Morphology of the upper airway epithelium. In: Andersen,
D.F.P.a.I. (Ed.) The nose: Upper airway physiology and the atmospheric environment. Elsevier, Amsterdam: 71-91.
13. Mathison S., Nagilla R., Kompella U.B. (1998) Nasal route for direct delivery of solutes to the central
nervous system: fact or fiction? Journal of Drug Targeting 5: 415-441.
14. Morrison E.E., Costanzo R.M. (1992) Morphology of the human olfactory epithelium. Journal of Comparative Neurology 297(1): 1-13.
15. Shipley M.T., Ennis M. (1996) Functional organization of olfactory system. Journal of Neurobiology 30:
123-176.
16. Vallee R.B., Bloom G.S. (1991) Mechanisms of fast and slow axonal transport. In: Cowan, W.M., Shooter
E.M., Stevens C.F., and Thompson R.F. (Eds.), Annual Review of Neuroscience, Vol. 14. Annual Reviews, Inc., Palo Alto, CA, USA; 59-92.
17. Fisher A.N., Brown K., Davis S.S., Parr G., Smith D. (1987) The effect of molecular size on the nasal
absorption of water-soluble compounds in the albino rat. Journal of Pharmacy and Pharmacology 39: 357-362.
18. Fisher A.N., Illum L., Davis S.S., Schacht E.H. (1992) Di-iodo-L-tyrosine-labelled dextrans as molecular size markers of nasal absorption in the albino rat. Journal of Pharmacy and Pharmacology 44: 550-554.
19. McMartin C., Hurchinson L.E.F., Hyde R., Peters G.E. (1987) Analysis of structure requirements for
the absorption of drugs and macromolecules from the nasal cavity. Journal of Pharmaceutical Sciences 76:
535-540.
20. Sakane T., Akizuki M., Yamashita S., Sekazi H., Nadai T. (1994) Direct transport from the rat nasal cavity to the cerebrospinal fluid: The relation to the dissociation of the drug. Journal of Pharmacy and
Pharmacology 46; 378-379.
21. Sakane T., Akizuki M., Yamashita S., Nadai T., Hashida M., Sekazi H. (1991) The transport of a drug to
the cerebrospinal fluid directly from the nasal cavity: The relation to the lipophilicity of the drug. Chemical
and Pharmaceutical Bulletin 39: 2456-2458.
22. Graff C.L., Pollack G.M. (2003) P-glycoprotein attenuates brain uptake of substrates after nasal
instillation. Pharmaceutical Research 20: 1225-1230.
23. Ying W., et al. (2007) Intranasal administration with NAD+ profoundly decreases brain injury in a ratmodel of transient focal ischemia. Frontiers in Bioscience 12: 2728-2734.
24. Wei G., et al. (2007) Intranasal administration of a PARG inhibitor profoundly decreases ischemic
brain injury. Frontiers in Bioscience 12: 4986-4996.