Response to Aschwin de Wolf's 'Evidence Based Cryonics'
In his article entitled ‘Evidence Based Cryonics’ Aschwin de Wolf unassailably argues that: “There is an urgent need to move from extrapolation based cryonics to evidence based cryonics. This will require a comprehensive research program aimed at creating realistic cryonics research models. It will also require vast improvements in the monitoring and evaluation of cryonics cases. The current debate should no longer be between advocates and opponents of standby and stabilization but about what stabilization procedures should be used by cryonics organizations given our current knowledge”.
Unfortunately, much of the rest of what he has to say is incomplete or lacks the necessary context required to allow for a fair and technically sound evaluation. Perhaps the brevity of the blog format was the reason for these shortcomings? In any event, I would like to comment on these remarks and provide a somewhat different perspective on the complex and important issues discussed in ‘Evidence Based Cryonics.’
The best place to start is to define what evidence based medicine is, and then proceed to attempt to describe what might constitute ‘evidenced based cryonics.’ Webster’s New World Medical Dictionary, 3rd Edition (2008) defines evidence-based medicine as, “the judicious use of the best current evidence in making decisions about the care of the individual patient. Evidence-based medicine (EBM) is mean to integrate clinical expertise with the best available research evidence and patient values. EBM was initially proposed by Dr. David Sackett and colleagues at McMasters University in Ontario, Canada.” Having defined what EBM is, the next question is, what constitutes “the best current evidence?”
The United States uses the U.S. Preventive Services Task Force (USPSTF) system for evaluating evidence about the effectiveness of medical interventions. The USPSTF classifies evidence in terms of reliability for use in decision making as follows:
* Level I: Evidence obtained from at least one properly designed randomized controlled trial.
* Level II-1: Evidence obtained from well-designed controlled trials without randomization.
* Level II-2: Evidence obtained from well-designed cohort or case-control analytic studies, preferably from more than one center or research group.
* Level II-3: Evidence obtained from multiple time series with or without the intervention. Dramatic results in uncontrolled trials might also be regarded as this type of evidence.
* Level III: Opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees.
* While beyond the scope of discussion here, it is worth noting (and referencing) the work of Guyatt, et al., and the GRADE Working Group in further defining what constitutes the quality and strength of scientific evidence; a formidable and controversial task (1- 6).
* To anyone knowledgeable in the areas of medicine applicable to human cryopatient stabilization and transport procedures (i.e., resuscitation/reanimatology, ischemia-reperfusion injury, solid organ preservation, deep hypothermic cardiopulmonary bypass and whole animal asanguineous perfusion) it will immediately be apparent that none of the 5 classes of evidence presented above can be directly applied to cryonics cases. Arguably, Level III evidence, the “opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees” might apply were there any acknowledged ‘respected authorities’ in the sphere of cryonics standby, stabilization or transport patient care. Alas, no such authorities, respected or otherwise, are currently ‘acknowledged’ to exist.
Thus, the first statement Aschwin makes in opening his article, “Cryonics patients can greatly benefit from rapid stabilization after pronouncement of legal death,” which he defines as “procedures that aim to rapidly restore blood circulation and drop the patient’s temperature” is itself unsupported by either conventional medical research or by cryonics research or case reporting using EBM criteria. If the information-theorertic criteria, as validated by ultrastructural preservation of the brain (7), or the demonstrated recovery of function of the brain are to be used as the gold standards for determining the efficacy of cryonics stabilization and transport procedures, then there currently exists no EBM quality (scientifically robust) data to support “restoration of blood circulation” following pronouncement of medico-legal death in cryopatients.
More specifically, assuming such an intervention is warranted, the question then becomes,’ under what circumstances and in which patients should it be applied?’ Is the patient with 30 minutes of post-arrest warm ischemia better off with simple external cooling followed by cryoprotective perfusion, as opposed to undergoing in-the-field reperfusion using closed chest cardiopulmonary support? What about the patient with profound peri-arrest hypoperfusion with evidence of failed or inadequate brain perfusion, such as the presence of fixed and unresponsive pupils for many minutes, or even for an hour or more, before cardiac arrest occurs and medico-legal death can be pronounced? At what point in the complex and difficult to quantify spectrum of warm ischemic injury should cardiopulmonary support be withheld? Or, given that the benefits of rapid post arrest cooling are unequivocally supported by Level II-2 and Level II-3 evidence from conventional medicine, should such support be modified to mitigate or prevent oxygen-driven reperfusion injury by carrying out CPS under anoxic conditions, and if so, under what circumstances and by what procedures? We have no rigorous answers to these questions and Aschwin is certainly on-point in calling for well designed, cryonics-appropriate studies to answer these and myriad other questions of great importance.
The problem is, as it has been since the inception of clinical cryonics in 1967, “what, if anything, do we do in the meantime?” Indeed, forty-two years later, we have little direct evidence even that cryoprotective perfusion results in superior conservation of identity-critical information under the real-world conditions encountered by today’s cryopatients than would be the case were they subjected to more timely straight freezing!
Is a patient who has suffered hours of warm ischemia better off simply being rapidly cooled and rendered into the solid state, as opposed to being subjected to 24, 48 or 72 hours of cold ischemia, followed by cryoprotective perfusion and freezing or vitrification? How do we even determine what the ultrastructural condition of a brain is following straight freezing? Freezing in the absence of fairly large amounts of colligative cryoprotectant agent(s) results in the collapse of tissue ultrastructure into dense channels of material, the structural condition of which it is currently not possible to determine by techniques such as transmission electron microscopy. Reaching conclusions based on the post-thaw ultrastructure (or lack thereof) of straight frozen tissue is complicated by the potentially myriad artifacts introduced during rewarming, thawing, fixation and embedding required to image tissue ultrastructure.
Given the extreme resource constraints that have historically been present in cryonics, and the lack of directly applicable mainstream medical research, the answer to the question of ‘what to do’ has been to apply reasoned extrapolation of high quality, peer-reviewed biomedical research to the care of the individual cryonics patient, and where possible, to conduct on-point in-house research to validate such armchair speculation.
It is important to point out that since the inception of cryonics in 1964, until approximately 1976, efforts to establish patient care protocols were a group effort between the then extant cryonics societies. The first of these efforts was organized by Robert Ettinger in 1966 and resulted in the protocol developed by Dante Brunol (8). Beginning in 1972, Fred and Linda Chamberlain, Art Quaife, Greg Fahy, Peter Gouras, M.D., Robert Ettinger, and I engaged in an extensive and largely public effort to reach a consensus about what should constitute a good standard of care for cryonics patients based upon extrapolation (and where feasible) experimental validation of findings in the peer-reviewed biomedical literature. This was done via extensive private correspondence, via publication of findings and recommendations in Manrise Technical Review and The Immortalist, as well as in the form of a detailed procedure manual for administering human cryopreservation entitled Instructions for the Induction of Solid State Hypothermia in Humans published by Fred and Linda Chamberlain and available, in part, on-line at: http://www.lifepact.com/mm/mrm001.htm (Readers interested in obtaining a copy of the full manual, for private use, may contact the author at ).
During the 1980s this effort continued and was both documented and subjected to review by the American Cryonics Society, Trans Time, Alcor and Cryovita Laboratories in the form of detailed technical presentations made at the annual Lake Tahoe Life Extension Conferences hosted by Fred and Linda Chamberlain’s Lake Tahoe Life Extension Festivals from 1979 to 1985[1.] An example of such disclosures is available at: http://www.lifepact.com/tahoe.htm.
In short, these efforts were public, largely collegial, and consisted of a best effort to apply insights from the scientific literature to human cryopatients. Furthermore, both Jerry Leaf and I made a sustained and detailed effort to document, by both presentations and publications, the outcomes achieved in detailed human cryopatient case reports (10-20) and in animal studies of post-cryopreservation ultrastructure, including those designed to reproduce conditions encountered under real-world conditions (21, 22).
These efforts resulted in a number of cryopatient stabilization protocols that incorporated multiple drugs to address the multiple mechanisms of ischemia-reperfusion injury as identified in the literature; an approach which Aschwin describes as administration of “an unorthodox number of medications to protect the brain and prevent impairment of circulation. While there are peer reviewed papers that combine a number of medications, there is no precedent in mainstream medicine or biomedical research in using such a large number of medications (in contemporary cryonics, medications protocol exceeds 12 different drugs and fluids).” This statement deserves further scrutiny.
Are poly-drug approaches to treatment unprecedented in medicine? As an example, let’s consider the case of a hyperkalemic hemodialysis patient who experiences cardiac arrest while preparations are being made for emergency hemodialysis. How many and what kind of medications will this patient likely receive in the setting of refractory cardiac arrest? Per the American Heart Association (AHA) Guidelines the patient will initially receive 1 mg epinephrine IV every 3 to 5 minutes during CPR. This may be substituted (after the first dose) with 40 IU of vasopressin IV. Since the patient is in aystole 1mg atropine IV is also given. Concurrent with the administration of these drugs the patient is given 30,000 IU of sodium heparin to allow for the institution of hemodialysis to definitively reduce the serum potassium level. The patient is given an unsuccessful 360 Joule shock at this point. Point of care evaluation of blood electrolytes discloses blood potassium of 12 mmol/L: a level that is incompatible with the return of spontaneous circulation. A decision is made to administer calcium chloride: 5 mL of 10% solution IV over 2 min and 2 amps (60 mlL) of 50% dextrose in water along with 10 IU regular insulin IV (glucose and insulin facilitate a transient profound cellular uptake of potassium from the interstitial and intravascular spaces). CPR is continued for 8 cycles and the patient is again defibrillated with a resulting non-perfusing rhythm consistent with hyperkalemic cardioplegia. CPR is continued while hemodialysis proceeds. After 4 minutes of hemodialysis a third defibrillation attempt is made with the result being coarse ventricular fibrillation. Following another unsuccessful defibrillation attempt, and confirmation by point of care testing that serum potassium has decreased to 7.8 mmol/L with blood pH at 6.95, 300 mg of amiodarone is given in addition to 1 mEq/kg sodium bicarbonate: by slow IV push; the latter to correct the acidosis that has resulted from prolonged CPR and dialysis with a low pH bicarbonate-acetate dialysate.. Following 5 additional cycles of CPR the patient is successfully defibrillated and recovers with a mild neurological deficit as a consequence of extended, low flow perfusion during CPR.
This patient, undergoing routine resuscitation from hyperkalemia cardiac arrest, has just received 9 discrete drugs, all of them indicated, and all of them within the current guidelines for the treatment of hyperkalemic cardiac arrest (23-24). Interestingly, none of these drugs was administered to ameliorate vital organ ischemia-reperfusion injury. The reason for this is that no such drugs are currently clinically available for this indication.
Similarly, patients undergoing acute fluid resuscitation and initial; treatment for septic shock may receive a dozen or more drugs including pressors, ionotropes, a vasodilator, 2-3 antibiotics, insulin, rAPC, and any ancillary drugs required to facilitate renal replacement therapy or mechanical ventilation (see: http://www.leedspicu.org/Documents/Septic%20shock.pdf). So, it is clearly not the case that, “there is no precedent in mainstream medicine” for a multimodal drug treatment approach to complex illness, since multi-drug interventions constitute the standard of care for resuscitation from both cardiac arrest and septic shock and increasingly serve as the backbone of a wide range of successful cancer chemotherapies.
However, it is the case that, at least until recently, multi-drug interventions in biomedical research to treat cerebral ischemia-reperfusion injury have been virtually nonexistent. This is beginning to change as there is increasing understanding of the complex, multifactorial nature of cerebral ischemia-reperfusion injury. Examples of this are the recent successful work of Buckberg, et al in recovering piglets from 90 minutes of deep hypothermic circulatory arrest using a protocol that employed 5 primary therapeutic drugs (plus leukodepletion using a Leukoguard filter in the arterial line during cardiopulmonary bypass) (25), the work of Liu, et al., demonstrating the effectiveness of a combination of cerebral blood flow promoting drugs and the administration of phenyl-N-tert-butyl-nitrone (a free radical inhibitor) and cyclosporine-A (a mitochondrial poration inhibitor) in improving 24 hour neurological outcome after 8 min of experimental normothermic cardiac arrest in pigs (26) and the work of Gupta, et al., combining melatonin and poly (ADP-ribose) polymerase inhibitors in a rat model of stroke – a study that employed 6 drugs in the most successfully treated group (27).Other research combining multiple drugs and other interventions, such as mild therapeutic hypothermia, have also shown positive results (28, 29).
Aschwin goes on to state, “The only existing justification for using current protocol reflects work done at Critical Care Research (CCR) in the 1990s. Although scattered reports exist about the effectiveness of this protocol in resuscitating dogs from up to 17 minutes of normothermic global ischemia, no detailed (peer reviewed) paper has been published about these experiments “
I do not know what is meant by the term “scattered reports” to describe disclosure of this work and would note that there have been two formal public disclosures, the first in the form of United States Patent 5700828 issued on 12/23/1997, and the second in the form of a public seminar which was subsequently distributed as a videotape: Darwin M, Harris, SB, Russell, SR, O’Farrell, Rasch, C, J, Pengelle, C, Fletcher, M. Routine Resuscitation of Dogs from 15-17 Minutes of Normothermic Ischemia (37.5°C) With Long Term Survival (>6 weeks). In: 21st Century Medicine Seminar on Recent Breakthroughs in Cryobiology and Resuscitation Research, Ontario, CA; 1998.
As the principal investigator on this study, I would be the first to agree that it is both regrettable and unacceptable that it has not been either peer reviewed or published. However, as I do not have access to either the primary or the reduced data from this study, I am personally powerless to remedy this situation. Further, I think it extremely unlikely either that I will be given access to this data, or that the results of this study will be published in any meaningful time frame, if at all, by those at CCR who control the study data.
The question thus arises as to whether the drugs identified in this study are of use, either singly or in combination, in the stabilization of cryonics patients? The only certain way to answer that question is to apply them in well designed animal models that closely approximate the spectrum of real-world conditions under which cryopatients eligible for cardiopulmonary support and pharmacological treatment of ischemia-reperfusion injury present for care. Such studies will take tens of thousands of dollars and several years to complete. So, again, the questions arise, ‘what do we do in the meantime ‘and ‘how do we judge the evidence that we use to justify any interventions we undertake?’
It is not possible to answer these questions without considering the specifics of the work in question. Aschwin states that, “in contemporary cryonics, medications protocol exceeds 12 different drugs and fluids” with the implication that the CCR canine resuscitation series (CRS) research was the source of these 12 drugs/fluids, presumably those described by Aschwin in his January 2007 article Human Cryopreservation Stabilization Medications (http://www.alcor.org/Library/html/stabilizationmeds.html).
In fact, the original CRS protocol included a total of 22 drugs!
o Hemodiluent: defined electrolyte-dextran-40 containing solution
o Hypertensives: 3 primary drugs, 1 secondary drug
o Buffer: tromethamine (THAM), 1 drug
o Antiglycemic: 1 drug
o Free radical inhibitors: 6 drugs
o Excitotoxicity Inhibitors: 3 drugs
o Ca++ Antagonists: 1 drug
o Bradykinin Inhibitor: 1 drug
o Leukotriene Antagonists/Inhibitors: 2 drugs
o COX I&II Inhibitors: 1 drug
o Phospholipase Inhibitor: 1 drug
o Antiplatelet: 1 drug
o PARS Inhibitor: 1 drug
o Metabolic Support: 2 drugs
TOTAL: 22 drugs
Of these, 6 drugs (not including the anticoagulant heparin, the hyperosmotic agent mannitol, the flow promoting agent dextran-40, and the buffer THAM, all of which were previously in use in cryonics) were retained in the protocol licensed by CCR to Alcor and to Suspended Animation, Inc. These drugs are s-methylthiourea (SMT), d-alpha tocopherol (Vitamin E), melatonin, alpha Phenyl t-Butyl Nitrone (PBN), kynurenine, and carprofen. How should the utility of these drugs be judged? The first step in such a process is to determine which patients might benefit based on the available information. By definition, only patients eligible for CPS can be treated, since effective use of all of these drugs requires thorough systemic distribution. Patients with 20 minutes or less of normothermic cardiac arrest are probably the only suitable candidates based on the limited ability of closed chest CPS to generate adequate pressure and flow over increasingly long intervals of cardiac arrest. Beyond this general criterion, it is necessary to consider the evidence for the utility of each drug individually, on the basis not only of the CCR study, but in the context of the published literature.
The patent which first discloses the core drugs used in the CCR protocol was United States Patent 5700828 which was filed on 12/07/1995. This is significant because two of the primary cerebroprotective drugs described in this patent, melatonin and PBN, had not been previously demonstrated to be neuroprotective in cerebral ischemia-reperfusion injury. It was not until 2003 that the first peer-reviewed paper documenting the effectiveness of melatonin in ischemia-reperfusion appeared (30) and not until 1999 that the effectiveness of PBN in cerebral ischemia was documented in the literature (31). Since these papers first appeared a vast literature supporting the effectiveness of melatonin in both focal and global cerebral ischemia-reperfusion injury has appeared, and the PBN analog NXY-059 was demonstrated as effective in a wide range of animal models of cerebral ischemic injury (32)., although the drug failed in a RCT of stroke (33).
The utility of vitamin E, mannitol and of dextran-40 in cerebral ischemia reperfusion injury predate the 1995 patent and are extensively documented in the cerebral resuscitation literature. There are few papers documenting the effectiveness of kynurenine, and no papers supporting the effectiveness of carprofen in cerebral ischemia-reperfusion injury, although there are many papers documenting the utility of other non-steroidal anti-inflammatory and NF-kappa B inhibiting drugs in cerebral resuscitation.
Should any or all of these drugs be applied to cryopatients? Aschwin raises a number of possible contraindications which merit consideration: “The lack of relevant published data to support the administration of large numbers of drugs…in cryonics is not just a matter of risking performing redundant procedures. A lot of time and resources are being spent in cryonics on obtaining and maintaining equipment and supplies for these procedures, in addition to the licensing fees paid to use some of these technologies and the training and recruiting of people to perform them. But perhaps the most troublesome problem is that the preparation and execution of these procedures during actual cryonics cases can seriously interfere with rapid and effective cardiopulmonary support and induction of hypothermia.”
It is clear from the foregoing that Aschwin considers immediate post arrest cooling in the presence of CPS to be an essential element of effective cryopatient stabilization. Unfortunately, the use of CPS in this setting carries with it the risks of return of consciousness (33) as well as the return of ‘signs of life’ such as agonal gasping (34, 35), spontaneous movement (36, 37) and even the return of spontaneous circulation. (38). This implies that the cryopatient undergoing CPS must be protected against these undesirable effects by pharmacological intervention. At a minimum, this means that intravenous (IV) or intraosseous (IO) vascular access must be established and at least 3 drugs must be administered (e.g., an anesthetic, a paralytic, and a cardioplegic). Thus, much of the skill, equipment and added personnel required to administer cerebroprotective drugs to cryopatients are, in fact, a requirement of delivering CPS assisted cooling. When Aschwin writes: “the preparation and execution of these procedures during actual cryonics cases can seriously interfere with rapid and effective cardiopulmonary support and induction of hypothermia” it is not clear what he means? Is it establishing IV or IO access, or the administration of a large number of drugs, or both that constitutes a threat to rapid post-arrest CPS and cooling?
CPS and vascular access must, necessarily, proceed together, with CPS (properly) trumping vascular access where any conflict occurs. It should also be noted that CPS, given in the absence of an effective pressor, and (in most cryopatients) volume expansion, will not achieve perfusion that is effective; either for supplying adequate cerebral blood flow to prevent ongoing ischemic injury, or to facilitate heat exchange. CPS implies not only vascular access and the attendant skills, complexity and hardware, but also the administration of at least half a dozen drugs in order to render it both safe and effective. Given this requirement, what are the additional burdens and costs of delivering cerebroprotective medication?
Currently, melatonin, PBN, vitamin E, and carprofen are combined into a single parenteral product by CCR (Vital-Oxy) which can be administered IV or IO via a stopcock manifold using a pressure infuser. Heparin (anticoagulant), vercuronium (paralytic), magnesium sulfate (cardioplegic) and the first dose of vasopressin (pressor) can similarly be combined to create a single parenteral product shortly before use and may be administered ‘push’ via the stopcock manifold. Dexrtran-40 and mannitol may also be combined into a single parenteral product with a total volume of ~550 mL which can also be given via pressure infuser and the stopcock manifold. Bolus, or continuous doses of vasopressin and THAM (buffer), can be given via the same stopcock manifold using battery operated infusion or syringe pumps.
The broader issue to be addressed is how these multiple medications may be given rapidly, accurately, and with the least use of personnel. Compact, battery operated infusion pumps for in-field use are now available, but they cost a fortune. The same is true of programmable, battery operated syringe pumps. I think the solution to this problem is to computerize it. A laptop computer should already be in use during cryopatient stabilization and transport to acquire data from the patient and it can and should be used to give the meds as well. One simple system for doing this would be to use pressure infusers, and syringes under pressure, with open/close line-clamp solenoids under computer control. Meds would be dispensed by the interval of solenoid opening; push meds would be a full open solenoid, and interval bolus meds would be given by briefly, and for a fixed time, opening the solenoid(s). This is an extremely simple system to implement from both the hardware and software standpoints. A schematic of this type of system is shown below:
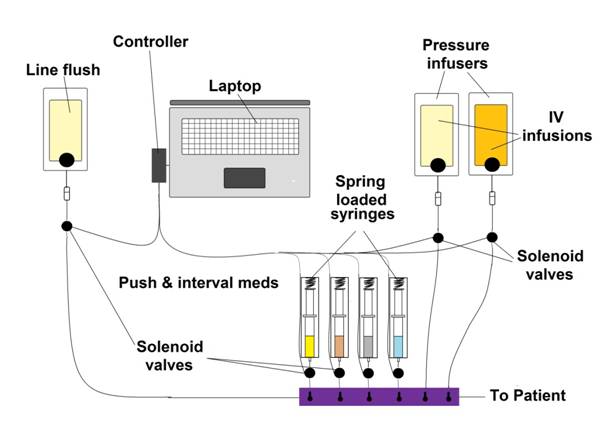
Of course, this presumes that the multidrug approach to cerebroprotection of the cryopatient is economically justified. I would be the first to agree that it is not necessary to pay costly licensing fees to derive most of the benefit from the CRS protocol. It is clear from reviewing the literature that the most widely validated and likely most potent drugs in this protocol (in the context of preventing ultrastructural injury secondary to ischemia-reperfusion injury) are melatonin, PBN[2], and, arguably, vitamin E. These are readily available molecules and may be used by any cryonics organization, absent licensing, on the basis of their documented protective effects in the literature. Other likely useful drugs such as dextran-40, THAM and mannitol have a long history of use in cryonics which predates the CCR research and these drugs may also be used at little cost (Darwin M. Transport Protocol for Cryonic Suspension of Humans, Fourth Edition. 1990, http://www.alcor.org/Library/html/1990manual.html).
In the nearly decade and a half that have elapsed since the CCR canine resuscitation series was undertaken many other promising experimental drugs for the inhibition or moderation of cerebral ischemia-reperfusion injury have emerged. I am in complete agreement with Aschwin that the best way to evaluate the potential utility of these drugs to cryopatients is in animal models that are truly relevant and which simulate the actual condition of cryonics patients who present for stabilization and transport. Such patients are typically suffering from extensive activation of the immune-inflammatory cascade, are often severely dehydrated or fluid overloaded, and invariably suffer from serious disturbances in cerebral microcirculation which begin hours or even days before medico-legal death is pronounced. As a consequence, these patients will typically have pre-arrest ischemic injury which will likely be compounded by post-arrest reperfusion. Evaluation of pharmacological interventions should, and indeed properly must be, carried out in animals models that reflect these facts.
Finally, Aschwin writes: “Even more complexity is introduced when cryonics organizations make an attempt to wash out the blood and substitute it with a universal organ preservation solution. The rationale for this procedure is found in conventional organ preservation and emergency medicine research. The question in organ preservation research is no longer whether hypothermic organs benefit from blood substitution with a synthetic solution, but what the ideal composition of such a solution should be. In emergency medicine research asanguineous hypothermic circulatory arrest is increasingly being investigated to stabilize trauma victims. But it is a major step from these developments to the practice of remote blood washout of ischemic patients with expected transport times of 24 hours or more. At present the only sure benefit of remote blood washout is that it enables more rapid cooling of the patient, a benefit that should not be underestimated. But when liquid ventilation becomes available to cryonics patients, rapid cooling rates will be possible without extracorporeal circulation.”
There can be no argument that blood washout followed by long delays to cryoprotective perfusion is deleterious (as currently practiced) on the basis of both clinical experience with cryopatients and recent unpublished animal research by Fahy, et al., of 21st Century Medicine (39). This practice should probably be abandoned until such time as effective solutions are developed for use in cryopatient transports. The statement that “when liquid ventilation becomes available to cryonics patients, rapid cooling rates will be possible without extracorporeal circulation,” is by no means assured. As the primary inventor of fractional tidal liquid assisted pulmonary cooling (40), I feel it is critical to point out that this technique has been validated only in the setting of healthy animals with spontaneous circulation. The reduced flow state attending external CPS and the typically severely injured lungs of the cryopatient present the twin challenges of greatly reduced blood flow coupled with greatly reduced pulmonary surface area (as a consequence of pre-existing or emergent lung injury; i.e., acute respiratory distress syndrome or acute lung injury resulting from closed chest CPS). which will dramatically reduce the efficacy of heat exchange achievable with this technique.
Once again, as Aschwin correctly notes in the context of pharmacological intervention, it is imperative that modalities developed for application in conventional clinical medicine be validated in the very different setting of the patient presenting for cryopreservation after succumbing to prolonged terminal illness – as well as the added insults of peri- and post-arrest systemic and cerebral ischemia.
REFERENCES:
1. Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ, for the GRADE Working Group. Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-926 or [pdf]
2. Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck-Ytter Y, Schünemann HJ; GRADE Working Group. Rating quality of evidence and strength of recommendations: What is “quality of evidence” and why is it important to clinicians? BMJ. 2008 May 3;336(7651):995-8
3. Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW Jr, Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH; GRADE Working Group. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ. 2008 May 17;336(7653):1106-10
4. Guyatt GH, Oxman AD, Kunz R, Jaeschke R, Helfand M, Liberati A, Vist GE, Schünemann HJ; GRADE working group. Rating quality of evidence and strength of recommendations: Incorporating considerations of resources use into grading recommendations. BMJ. 2008 May 24;336(7654):1170-3
5. Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, Schünemann HJ; GRADE Working Group. Rating quality of evidence and strength of recommendations: Going from evidence to recommendations. BMJ. 2008 May 10;336(7652):1049-51
6. Jaeschke R, Guyatt GH, Dellinger P, Schünemann H, Levy MM, Kunz R, Norris S, Bion J; GRADE working group. Use of GRADE grid to reach decisions on clinical practice guidelines when consensus is elusive.
BMJ. 2008 Jul 31;337:a744.
7. Merkle RC: The technical feasibility of cryonics. Med Hypotheses. 1992, 39:6-16.
8. Robert F. Nelson & Sandra Stanley: We Froze The First Man, Dell Publishing Company, New York, 1968, pp. 136-56.
9. Leaf, JD, Cryonic suspension of Sam Berkowitz technical report. Long Life Magazine, 3:(2), March/April, 1979, pp. 30-35.
10. Leaf, JD, Case study: K.V.M. suspension, Cryonics, August 1981, pp. 8-18.
11. Leaf, JD, Quaife A, Case study in neurosuspension. Cryonics. 16 November, 1981 pp. 21-28.
12. Leaf, JD, Darwin, M, Hixon, H, Case report: two consecutive suspensions, a comparative study in experimental suspended animation. Cryonics, August 1981, pp. 8-18.
13. Darwin, MG, Leaf, JD, Hixon, HL, Cryonic suspension case report: A-1133, 08 June, 1987, http://www.alcor.org/Library/pdfs/AlcorCaseA1133.pdf.
14. Darwin, MG, Cryonic suspension case report: A-1108, 08May, 1988, unpublished technical case report of the Alcor Life Extension Foundation
15. Darwin, MG, Cryonic suspension case report: A-1165, 08 October, 1988, unpublished technical case report of the Alcor Life Extension Foundation
16. Darwin, MG, Cryonic suspension case report: A-1169, 21 March 1989, unpublished technical case report of the Alcor Life Extension Foundation
17. Darwin, MG, Cryopreservation patient case report: Arlene Francis Fried, A-1049, 06/09/1990, http://www.alcor.org/Library/html/fried.html.
18. Darwin, MG, Cryopreservation case report: Jerome Butler White, 02-05-1994, unpublished, available from the author upon request.
19. Darwin, MG, Cryopreservation case report: Richard Putnam Marsh, 05-06-1994, unpublished, available from the author upon request.
20. Darwin, MG Cryopreservation of James Gallagher CryoCare patient #C-2150, CryoCare Report Number 6, January 1996, and CryoCare Report Number 9, October 1996, http://www.alcor.org/Library/html/casereportC2150.html.
21. Federowicz (Darwin), MG and Leaf JD, Cryoprotective perfusion and freezing of the Ischemic and nonischemic cat. Cryonics, issue 30, p.14, 1983.
22. Federowicz (Darwin), MG and Leaf JD,. The Effects of Cryopreservation on the Cat. Research reported on Cryonet, December 1992.
23. Part 7.2: Management of Cardiac Arrest, Circulation 2005;112;IV-58-IV-66; originally published online Nov 28, 2005, http://circ.ahajournals.org/cgi/content/full/112/24_suppl/IV-58.
24. Alfonzo, AVM, Simpson, K, Deighan, C, Campbell, S, Fox, J. Modifications to advanced life support in renal failure. RESUS-3067; No. of Pages 17, in press.
25. Buckberg, GJ, Deep hypothermic circulatory arrest and global reperfusion injury: Avoidance by making a pump prime reperfusate—A new concept. J Thoracic and Cardiovasc Surg. 2003;125(3); 625-632.
26. Gupta,S, Kaul, CL, Sharma, S. Neuroprotective effect of combination of poly (ADP-ribose) polymerase inhibitor and antioxidant in middle cerebral artery occlusion induced focal ischemia in rats Neurological Research,26;2004:103-107.
27. LIiu, XL, Nozaria, A, Basu, S, Ronquist, G, Rubertsson, S, Wiklund, L. Neurological outcome after experimental cardiopulmonary resuscitation: a result of delayed and potentially treatable neuronal injury? Acta anaesthesiologica scandinavica,46,(5) 2002:.537-546.
28. Schmid-Elsaesser R, Hungerhuber E, Zausinger S, Baethmann A, Reulen HJ. Combination drug therapy and mild hypothermia: A promising treatment strategy for reversible, focal cerebral ischemia. Stroke 1999, 30: 1891–1899
29. Spinnewyn B, Cornet S, Auguet M, Chabrier PE. Synergistic protective effects of antioxidant and nitric oxide synthase inhibitors in transient focal ischemia. J Cereb Blood Flow Metab 1999; 19:139–14.
30. Reiter RJ, Tan DX. Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res. 58:10–19, 2003.
31. Shuaib A, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Diener HC, Ashwood T,Wasiewski WW, Emeribe U; NXY-059 for the treatment of acute ischemic stroke, N Engl J Med. 2007 Aug 9;357(6):562-71.
32. Siesjö BK, Elmer E, Janelidze S, Keep M, Kristian T, Ouyang,YB et al. Role and mechanisms of secondary mitochondrial failure. Acta Neurochir (Suppl) 1999: 73: 7–13.
33. Darwin, MG, Leaf, JD, Hixon, H, Neuropreservation of Alcor Patient A-1068. http://www.alcor.org/Library/html/casereport8504.html#part2
34. Clark J, Larsen, MP, Culley, LL, Graves, JR, Eisenberg, MS. Incidence of agonal respirations in sudden cardiac arrest. Ann Emerg Med 1992;21(12):1464-7.
35. Rea TD. Agonal respirations during cardiac arrest. Curr Opin Crit Care 2005;11(3):188-91.
36. Jain S, DeGeorgia, M. Brain death-associated reflexes and automatisms. Neurocrit Care 2005;3(2):122-6.
37. Maurino, SJ, Saizar, R,.Bueri.J, Frequency of spinal reflex movements in brain-dead patients. The American Journal of Medicine, 2004, 118(3):311-314;36.
38. Vukmir R, Bircher, N, Radovsky, A, Safar, P. Sodium bicarbonate may improve outcome in dogs with brief or prolonged cardiac arrest. Crit Care Med 1995;23:515-22.
39. Fahy, GM The Whole-Body Vitrification Project at 21st Century Medicine. In the Suspended Animation conference held in Fort Lauderdale, Florida, from May 18th through May 20th May 2007.
40. Federowicz (Darwin), MG, Russell, SR, Harris, SB, Mixed-mode liquid ventilation gas and heat exchange. United States Patent 6,694,977, published 24 February, 2004, http://www.freepatentsonline.com/6694977.html.
ENDNOTES:
[1] The Cryonics Institute declined repeated invitations to participate in these colloquiums.
[2] It is important to note that the PBN used in the CCR study was prepared by dissolving it in boiling water with concurrent microwave heating in the presence of atmospheric oxygen. This is very likely significant because such handling would inevitably create breakdown products of PBN, such as NtBHA and its oxidation product the spin-trap MNP. As Proctor, et al., have pointed out, (Peter H. Proctor and Lynsey P. Tamborello, SAINT-I Worked, But the Neuroprotectant Is Not NXY-059, Stroke 2007 38:e109; published online before print August 23 2007.) it is possible that the failure of NXY-059 in the SAINT-II trial was due to the fact that material used in this trial differed from that used in the successful SAINT-I trial in that it was stabilized and protected against oxidation. It may well be that it is not PBN, per se, that is cerebroprotective, but rather its oxidation and/or break-down products.