CPR and the breath of death?
And the Lord God formed man of the dust of the ground, and breathed into his nostrils the breath of life; and man became a living soul. Genesis 2:7
For breath is life, and if you breathe well you will live long on earth. – Sanskrit Proverb
In the Beginning…
Since the beginning of modern resuscitation over 40 years ago the sequence of interventions, and to a great extent their importance, has been determined by the ABCs of cardiopulmonary resuscitation (CPR): Airway, Breathing and Circulation. [1] Without breath there is no life and the most obviously and easily detected sign that life is fleeing is the absence of breath. What’s more, in many in whom breathing has newly ceased, simply opening the airway, or giving a single rescue breath will restore spontaneous respiration. We are told that breath is life and there is great truth in this saying.
Negative Pressure Ventilation
The physiology of the vertebrate chest is a truly amazing thing and it can take very bright men years to master its implications. One of the hardest concepts for me to grasp was that under normal conditions the pressure inside the pleural space (the space around the lungs) and the mediastinal spaces (the space that surrounds the heart and the great vessels of the chest) is always negative relative to both the atmospheric pressure and the pressure in the rest of the body (more on this later). [2] In other words, there is always a slight vacuum in those parts of the chest. Mostly this is a result of the fact that we breathe using our diaphragms and the muscles of our chest wall to create a low grade vacuum inside the chest into which air rushes via the trachea to fill our lungs. When we relax those muscles, the natural elasticity, or recoil of the chest wall and diaphragm acts to squeeze the air out [I], and the cycle is then repeated. We thus breathe by means of negative pressure ventilation.
Naturally enough, when medicine began to try to restore breathing when it had ceased, most of the attempts that were made were attempts to simulate the natural process whereby air is moved in and out of the lungs; principally by negative intrathoracic pressure. [3]
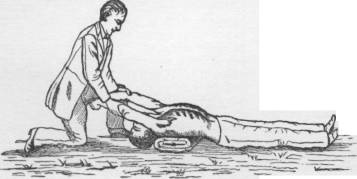
Above: an illustration of the Silvester method of artificial respiration circa 1880. (Silvester HR., A new method of resuscitating still-born children, and of restoring the persons apparently drowned or dead. BMJ 1858;2:576-9.)
Understandably, trying to mimic the complex action of the diaphragm and accessory muscle of the chest wall in normal negative pressure ventilation were difficult, cumbersome and often ineffective. By 1959 rescue breathing using the mouth-to-mouth technique had become the mandated medical standard. [4]
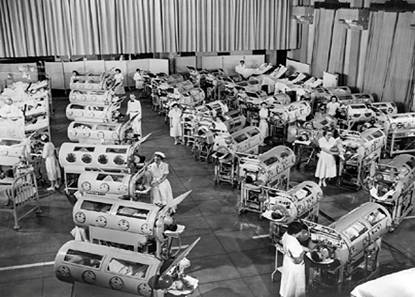
There is no question that mouth-to-mouth resuscitation is vastly more effective than the medically endorsed methods that preceded it. What’s more, the class of ventilation to which mouth-to-mouth belongs, intermittent positive pressure ventilation (IPPV), has become essentially the only modality for assisting or replacing breathing in humans. IPPV long ago displaced negative pressure ventilation (NPV) in the form of the iron lung and the cuirass shortly after polio was vanquished in the 1950s.
Below: Polio patients suffering from respiratory paralysis in iron lungs during an epidemic in the early 1950s.
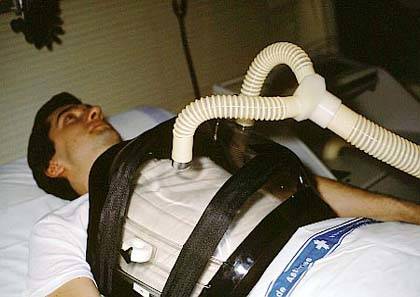
Below: A contemporary cuirass ventilator; this new breed of machines uses a biphasic approach that employs negative pressure on inspiration and positive pressure assisted exhalation. The Hayek biphasic cuirass ventilator is capable of delivering higher tidal volumes (negative inspiratory tidal volume and positive expiratory tidal volume), greatly increased frequency of ‘breaths’ from 6 to 1200CPM, and allows control of the inhalation to exhalation ratio without depending upon the passive recoil of the patient’s chest. Courtesy of United Hayek Medical: http://www.unitedhayek.com/products/mrtx
While IPPV has many advantages over NPV, it is un-physiologic in one way that turns out to be of crucial importance in the setting of CPR. IPPV dramatically reduces the return flow of blood from the body to heart. The physics and physiology of this are complex, and even the shortest and most lucid tutorials run to many pages of text; often studded with equations and difficult to interpret graphs and charts. Among the many reasons IPPV’s effects are so complex in the human with a beating heart is that the body can respond to changes in venous blood flow, ventricular volume, and so on by dynamically adapting; heart rate can be increased, vascular tone can be altered, and even the amount of fluid retained by the body can be altered. [5]
The Danger of Positive Pressure Ventilation in CPR
For the patient in cardiac arrest none of these adaptive changes is possible. That makes discussion of the problems posed by IPPV much simpler, while at the same time making the adverse consequences more serious and potentially life threatening.
To understand why IPPV is so dangerous in the setting of CPR it is necessary to understand the concept of preload. Most simply, preload can be defined as “the load to which a muscle is subjected before shortening.” If this doesn’t leap out at as a point of great significance it is because this definition needs to be understood in connection with a physiological principle named after the two great physiologists who discovered it, the Frank-Starling Law of the Heart [II]. The Frank-Starling law of the heart (also known as Starling’s law or the Frank-Starling mechanism) states that the greater the volume of blood entering the heart during diastole (the period between cardiac contractions or the end-diastolic volume), the greater the volume of blood ejected during systolic contraction (stroke volume). This may seem pretty obvious: what you put into the heart before it contracts determines what you’ll get out of it after contraction is complete. But, as it turns out, this relationship is rather more complex than simple double entry bookkeeping would suggest.
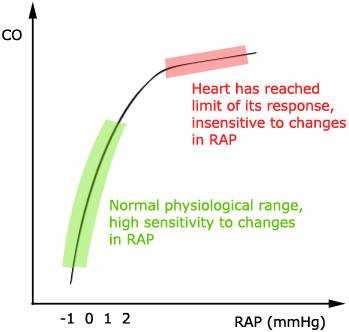
Above: Frank-Starling Cardiac Function Curve. Courtesy of Wikimedia Commons.
In the diagram above the Y-axis shows the cardiac output and the X-axis shows right atrial pressure. While the above diagram shows only one line, a classic Frank-Starling plot often shows three separate lines, each roughly the same shape, one atop of each other, to illustrate that shifts on the same line indicate a change in preload, while shifts from one line to another indicate a change in afterload or contractility. This allows the cardiac output to be synchronized with the venous return and with the cardiac output; without depending upon external regulation to make changes. For our purposes this diagram does the job in that it shows that the relationship between venous return from the body to the right heart has a powerful effect not only on how much the blood the heart pumps, but also on the efficiency with which it pumps that blood.
As the heart is increasingly loaded with blood it becomes better and better at pumping it out to the body (up to a point) by contracting more forcefully and emptying the cardiac chambers more completely with each heartbeat. However, as the graph also shows, there is a threshold of filling or stretching of the ventricles beyond which further filling, or preload, causes no further improvement in cardiac output and (not shown) ultimately causes a decrease in pumping efficiency with a corresponding decrease in cardiac output.
Special attention needs to be paid to the Y-axis on the graph because the huge change in cardiac output shown on X-axis happens due to a change in right atrial pressure of just 3 mm Hg, or 4.1 cm H20. [6]
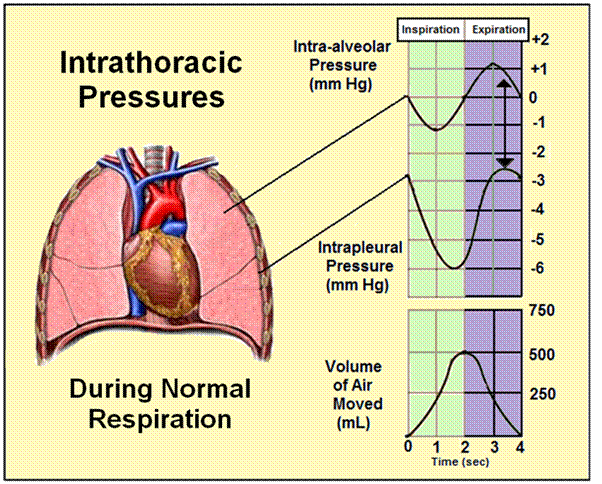
To put this change in right atrial pressure in context it is only necessary to look at the graphic above to realize that a change in pressure of 3-4 mm Hg is the maximum that occurs over the course of the whole respiratory cycle. Even more importantly, as is also illustrated above, is the seeming impossibility that the pressure inside the pleural mediastinal spaces, the latter of which which contains the heart and the great vessels, is always negative throughout the entire respiratory cycle. Put another way, the areas inside the chest that conduct blood away from and back to the heart always operate under a low vacuum: ~ -6.8 cm H2O during the height of inspiration, and ~ -2.0 cm H2O at the end of exhalation. [7] This continuous negative intrathoracic pressure serves to aid venous (and lymphatic) return from the body to the heart and thus to facilitate preload and optimize cardiac output.
By contrast, a quick glance at the graphic below, which is an actual pleural pressure tracing from a patient undergoing mechanical IPPV, shows that the thoracic viscera, including the vena cavae and right heart, are subjected to positive pressures throughout the respiratory cycle which, at their peak, are typically 30 to 40 times higher than those experienced during normal (negative) pressure breathing! In fact, the situation is even worse than it seems at first glance because not only does the intrathoracic pressure never go to zero (let alone to a negative value), it is deliberately kept positive, in this case by about 8 cm H2O. This is done because in the absence of lung expansion under the influence of the intrathoracic vacuum, the alveoli, the small air sacs of the lungs, begin to collapse and stick to each other. Collapsed alveoli are very difficult to re-open and restore ventilation to. Thus, it is necessary to keep them inflated at the end of exhalation by maintaining some positive pressure in the airways. Since this pressure is applied and maintained at the end of a breath (and between breaths) it is called positive end expiratory pressure, or PEEP, for short. [8]
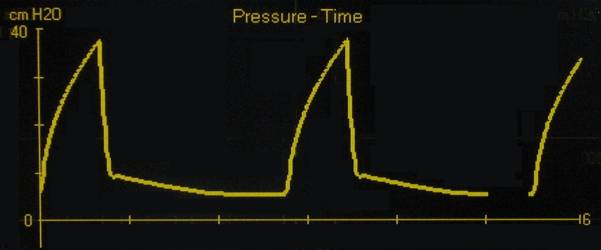
Intrapleural pressure tracing of a patient with acute respiratory distress syndrome unergoing intermittent positive pressure mechanical ventilation wih a PEEP of 8 cm H2O
The graphic below shows the effect of only 3 cm of H2O PEEP on blood flow from the head and upper body through the superior vena cava (SVC). Just 3 cm H2O of PEEP reduces superior vena cava (SVC) blood flow by 25%! [9] A patient with a spontaneously beating heart can (and does) usually compensate for this reduction in return blood flow from the body in many ways, none of which are available to the patient in cardiac arrest.
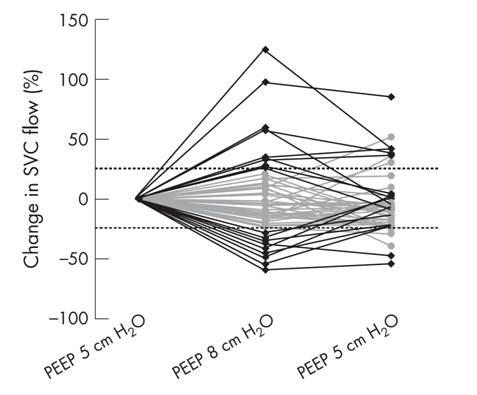
The effect of a 3cm H2O water change in PEEP on the flow of venous blood returning to the right heart from the upper body via the superior vena cava. [9]
During conventional closed chest CPR, or in the case of the cryonics patient cardiopulmonary support (CPS), the patient experiences truly massive increases in intrathoracic pressure that last longer and reach extremes never seen in IPPV. The largest and most deleterious source of this pressure results from the physical compression of the thoracic contents during the down-stroke of chest compressions. This raises the intrathoracic pressure to between 80 and 110 cm H2O for roughly 50% of the CPR duty cycle – in other words, the pressure inside the chest is about the same as the normal averaged (mean) blood pressure in a healthy adult for half of the time the patient is undergoing CPR/S! Added to this pressure is the additional pressure of IPPV and the PEEP (usually 5 cm H2O) required to keep the small airways open. The effects of these profoundly un-physiologic pressures on the return of venous blood to the heart and consequently on cardiac output during CPR are devastating. [10]
Overcoming Increased Intrathoracic Pressure and Preserving Cardiac Output
Following the development of active compression decompression CPR (ACD-CPR) by Cohen, et al., in 1992 [11] the critical importance of maintaining negative intrathoracic pressure during the decompression phase of the CPR duty cycle has become increasingly understood. [12, 13, 14] There is a rapidly growing body of both animal and clinical CPR research documenting improved survival and decreased neurological morbidity when the intrathoracic pressure is kept negative during the decompression (release of chest compression) phase of CPR by the use of inspiratory impedance threshold devices, such as the ResQPod, and ACD-CPR. [15, 17, 18] An instructive video demonstration of how the ResQPod works can be seen at: http://www.advancedcirculatory.com/
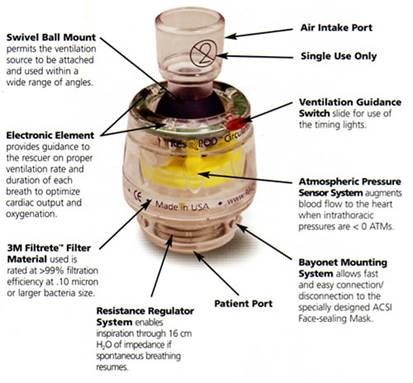
ResQPod impedance threshold device. Courtesy of Advanced Circulatory systems, Inc. http://www.advancedcirculatory.com/
Similarly, there is accumulating evidence that the increased intrathoracic pressure that results from excessive IPPV during CPR dramatically reduces cardiac output (CO) and causes increased morbidity and mortality. [19, 20, 21].
In 2004, Yannopoulos, et al., reported the development of a device which allows for the continuous application of negative intrathoracic pressure by applying controlled suction to the airway. [22] This device combines an ITD with a vacuum source and a negative pressure regulating valve to maintain an intrathoracic vacuum of between 5 to10 mm Hg (6.8 to 13.6 cm H2O), while allowing IPPV to proceed normally. This device, called the intrathoracic pressure regulator (ITPR) allows IPPV to be delivered as needed during ACD-CPR, while maintaining negative intrathoracic pressure when PPV is not being administered. The device effectively restores the intrathoracic space to its natural state as a low negative pressure (vacuum) chamber; increasing venous return from the body and consequently increasing preload and cardiac output. The ITPR also markedly increases coronary perfusion pressure (CPP) [III] while at the same time decreasing intracranial pressure (ICP). In CPR ICP is typically elevated from the basal value of 12-16 mm Hg to 22-30 mm Hg (as the result of pressure transmission by blood in non-valved veins and by transmission of intrathoracic pressure via the cerebrospinal fluid) further compromising already inadequate cerebral perfusion. [23] Reduction of ICP during CPR has been shown to improve both survival and neurological outcome in an animal model of CPR. [24]
Prototype ITPR (Advanced Circulatory Systems, Inc.) in position in a typical bag-vale resuscitator – ET tube set-up.
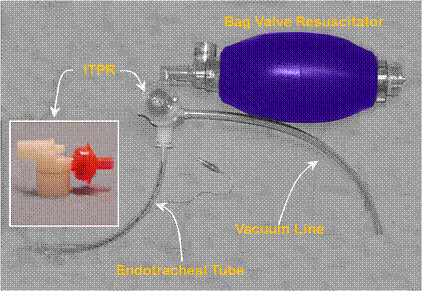
The ITPR has been shown to dramatically improve gas exchange, hemodynamics, cardiac output, vital organ perfusion, and short-term survival during ventricular fibrillation (VF) cardiac arrest in a porcine model of sudden cardiac arrest and CPR. [25] The ITPR is able to not only overcome the high intrathoracic pressures associated with CPR (45 to 55 mmHg or ~61 to 75 cmH20 [26]) but to both create and sustain negative intrathoracic pressure (determined indirectly by measuring the ET tube pressure) continuously during prolonged periods of ITPR-CPR, even in the presence of induced hypovolemia. [27] In hemorrhaged (hypovolemic) pigs, Yannopoulos, et al., were able to sustain CPP at >15 mm Hg (the accepted threshold for successful defibrillation in human sudden cardiac arrest) and the isovolemic VF animals in the study maintained CPP at >25 mm Hg throughout the full 15 minutes of ITPR-CPR. In both groups, the ETCO2 was consistently maintained above 25 mm Hg, and the 1-hour survival was 100%, as contrasted with 10% in control animals receiving AHA standard CPR (P = 0.0001).
By comparison, after 3 minutes of conventional CPR the control animals had a mean coronary perfusion pressure of less than 15 mm Hg and all had developed pseudo-respiratory alkalosis indicative of the ventilation/perfusion mismatch of standard CPR. [28] Blood gases in VF animals were strikingly preserved during ITPR-CPR; paO2, which was 96±2 mm Hg at baseline, was 214±12.37 mm Hg after 10 min and 198±6.75 mm Hg after 15 min of ITPR-CPR. These findings would seem to suggest that ITPR-CPR may be reducing or eliminating the pulmonary edema that accompanies CPR and the high intrathoracic (and thus pulmonary arterial and venous pressures) generated during CPR. ITPR is similarly effective at improving both hemodynamics and survival in a swine model of severe hypovolemic hypotension. [29]
The Breath of Death?
However, even with the use of the ITPR, each ventilation transiently raises intrathoracic pressure and decreases CO. In the setting of CPR/S it might be said that each breath is potentially a ‘breath of death’ in terms of its impact on perfusion.
In 2007 the author began experimenting with ways to eliminate tidal ventilation during CPR/S using simple mechanical systems to simulate the lungs and thorax. Based on this preliminary work it appears that it will be possible to eliminate tidal ventilations completely while at the same time maintaining a negative intrathoracic pressure during the non-compression portion of the CPR/S duty cycle. This is possible by the simple expedient of connecting the patient’s airway to a regulated vacuum source while at the same time delivering the desired minute volume of ventilating gas to the carina of the trachea. This scheme is illustrated in the schematic below.
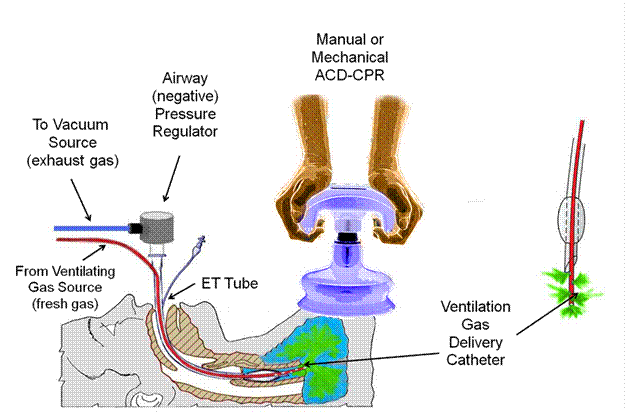
Above: Experimental implementation of continuous negative pressure ventilation in CPR/S. A conventional endotracheal tube is modified by passing a fenestrated suction catheter down the lumen to the level of the carina through a side opening in the tube through which ventilating gas is continuously delivered at the desired minute ventilation volume. Negative intrathoracic pressure and removal of waste gas is achieved by applying continuous, regulated suction to the 15 mm adapter on the end of the ET tube. Movement of gas to and from the distal airways is achieved by the action of the ACD chest compressor-de-compressor operating at 100 cycles/min on the ventral thorax.
Continuous Non-Tidal Negative Pressure Ventilation
A (negative) airway pressure regulator is attached to the 15 mm connector of a modified endotracheal tube. Continuous negative airway pressure is generated by connecting the airway pressure regulator to a vacuum/suction source. Ventilating gas of the desired composition (i.e., oxygen concentration, therapeutic additives, etc.) is continuously delivered to the patient’s lungs by a ventilation gas delivery catheter that can be advanced or withdrawn through the lumen of the ET tube. The ideal position for the ventilation gas delivery catheter is at the point where the trachea bifurcates into the main-stem bronchi; the carina. Movement of the ventilating gas to the distal airways is achieved by the dynamically varying force on the chest resulting from cardiac compressions, with or without active decompressions. The desired minute volume is determined by the flow rate of ventilating gas delivered to the carina.
In cases where pulmonary edema is preventing adequate gas exchange and it is deemed necessary to apply positive intrathoracic pressure (at the expense of cardiac output) it is easy to do this by adjusting the pressure on the airway pressure regulator to the desired positive pressure setting; as would be done with the application of positive end expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) as is done for the treatment of pulmonary edema by a different means with the Boussignac tube. [30, 31]
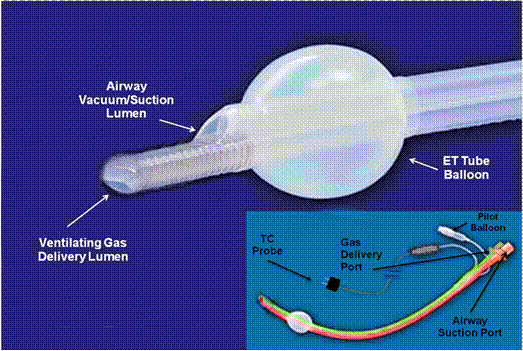
The illustration above shows a possible configuration of a dual-lumen endotracheal tube that would accomplish continuous non-tidal negative pressure ventilation in conjunction with an attached airway pressure control valve.
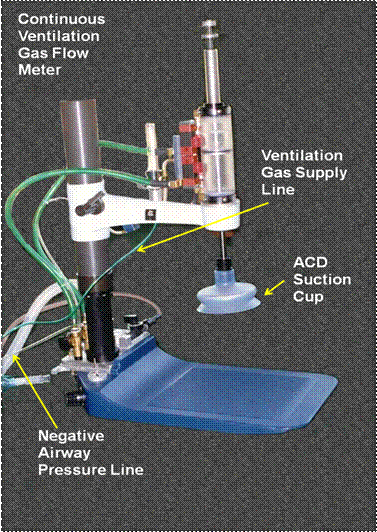
Prototype Active Comnpression-Decompression square-wave HLR constructed to the author’s specifications by Michigan Instruments, Inc., of Grand Rapids, MI. The device incorporates a continuous ventilation gas flow meter (red arrow) which allows for delivery of air blended oxygen at the desired oxygen concentration and minute volume.
In 1996 a prototype heart–lung resuscitator capable of delivering non-tidal continuous negative pressure ventilation in conjunction with ACD-square wave-CPR was fabricated by Michigan instruments of Grand Rapids, MI (shown above). Unfortunately, this device was never evaluated in a canine or porcine model of CPR in cardiac arrest. Bench testing with a sophisticated test lung did demonstrate that the unit could deliver adequate minute ventilation while maintaining operator specified negative (or positive) intrathoracic pressure throughout the decompression phase of the CPR/S duty cycle.
If animal research confirms that this mode of ventilation (which is an extension of the ITPR) is both safe and effective it should be possible to apply it to cryonics patients without the years-long wait for regulatory approval. In the case of CPR in the clinical environment, non-tidal continuous negative pressure ventilation using this scheme will likely remain one of the many intriguing and potentially useful ideas in CPR that await another time and place, or perhaps more accurately, a different universe in order to find application.
The End of the Thumper Era?
With clinical advent of ACD-CPR, and the experimental debut of the ITPR and negative intrathoracic pressure CPR delivered via the trachea, it would seem that the days of the Michigan Instruments, Inc. (MII) Thumper CPR devices, which have been the dominant technology used in cryonics transport operations, are over. Despite the very low cost of used Thumper HLRs ($150 to $500 US) the capability of doubling or trebling cardiac output (and sometimes quadrupling cerebral blood flow during CPR) would seem to mandate the expense of either custom fabricated MII equipment, or purchase of a LUCAS or LUCAS 2 device ($20,000 US).
However, the reality is that the ITPR offers the older generations of conventional CPR machines a new lease on life. The raison d’être for ‘suction cup’ CPR is to create negative intrathoracic pressure by forcefully pulling up on the ventral chest wall between compressions. This method of creating negative intrathoracic pressure was developed as a consequence of the case of a successful resuscitation from cardiac arrest having been carried out by a layman using a lavatory toilet plunger; not as a result of systematic academic or medical laboratory investigation. [32]
It is the principle that is important, and in this case the principle is negative intrathoracic pressure; something that can be applied by surrounding the thorax with a vacuum in a cuirass, or by using a suction cup to decompress the chest wall. Importantly, it can also be accomplished by the ITPR and without the use of complex pneumatic or electromechanical machinery to move a suction cup to and fro. [IV] The ITPR and continuous negative pressure ventilation suggest a number of novel ways that may allow for the continued use of costly first and second generation mechanical CPR equipment – perhaps with even greater efficacy than is possible with the latest generation HLRs. But, alas, that is a subject for another article.
REFERENCES
1) Eisenberg MS., Cardiac Arrest. The science and practice of resuscitation medicine. In: Paradis NA, Halperin HR, Nowak RM, editors. The quest to reverse sudden death: a history of cardiopulmonary resuscitation. Baltimore: Williams and Wilkins; 1996.
2) West, JB., Pulmonary Physiology and Pathophysiology, Lippincott Williams & Wilkins, Philadelphia, 2000.
3) Baskett, FF., The Holger Nielsen Method of Artificial Respiration Resuscitation (2007) 74, 403-405.
4) Acierno LJ, Worrell LT., Peter Safar: father of modern cardiopulmonary resuscitation. Clin Cardiol. 2007 Jan;30(1):52-4.
5) Luecke , T, Pelosi, P., Clinical review: Positive end-expiratory pressure and cardiac output. Critical Care 2005, 9:607-621 (DOI 10.1186/cc3877).
6) JE, A.D., Carlson CJ, et al., Continuous positive-pressure ventilation decreases right and left ventricular end diastolic volumes in the dog. Circ Res, 1980. 46: p. 125-132.
7) West, JB., Pulmonary Physiology and Pathophysiology, Lippincott Williams & Wilkins, Philadelphia, 2000.
8) Acosta, E, Varon, S. The Use of Positive End-Expiratory Pressure in Mechanical Ventilation. Critical Care Clinics, Volume 23, Issue 2, Pages 251-261.
9) de Waal, KA, Evans, N. Osborn, DA, Kluckow , K, Cardiorespiratory effects of changes in end expiratory pressure in ventilated newborns. Arch Dis Child Fetal Neonatal Ed 2007;92:F444–F448. doi: 10.1136/adc.2006.103929.
10) Jellinek, H., Krenn, H, Oczenski, W, et al., Influence of positive airway pressure on the pressure gradient for venous return in humans. J Appl Physiol, 2000. 88: p. 926-932.
11) Cohen, T., Tucker, KJ, Redberg, RF, Lurie, KG, Chin, MC, Dutton, JP, Scheinman, MM., Active compression-decompression resuscitation: a novel method of cardiopulmonary resuscitation. Am Heart J, 1992. 124: p. 1145-50.
12) Jellinek, H., Krenn, H, Oczenski, W, et al., Influence of positive airway pressure on the pressure gradient for venous return in humans. J Appl Physiol, 2000. 88: p. 926-932.
13) Aufderheide, T., Sigurdsson, G, Pirrallo, RG, et al. , Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation, 2004. 109: p. 1960-1965.
14) Cheifetz, I., Craig, DM, Quick, G, et al., Increasing tidal volumes and pulmonary overdistention adversely affect pulmonary vascular mechanics and cardiac output in a pediatric swine model. Crit Care Med, 1998. 26: p. 710-716.
15) Cabrini, L., Beccaria, P, Landoni, G, Biondi-Zoccai, GG, Sheiban, I, Cristofolini, M, Fochi, O, Maj, G, Zangrillo, A., Impact of impedance threshold devices on cardiopulmonary resuscitation: a systematic review and meta-analysis of randomized controlled studies. Crit Care Med, 2008. 36: p. 1625-32.
16) Plaisance, P., Lurie, K, Payen, D., Inspiratory impedance during active compression decompression cardiopulmonary resuscitation: a randomized evaluation in patients in cardiac arrest. . Circulation, 2000. 10: p. 989-994.
17) Wolcke, B., Mauer, DK, Schoefmann, MF, Teichmann, H, Provo, TA, Lindner. KH, Dick WF, Aeppli D, Lurie KG., Comparison of standard cardiopulmonary resuscitation versus the combination of active compression-decompression cardiopulmonary resuscitation and an inspiratory threshold device for out-of-hospital cardiac arrest. Circulation, 2003. 108: p. 2201-2205.
18) Lurie, K., Voelckel, W, Plaisance, P, et al., Use of an inspiratory impedance threshold valve during cardiopulmonary resuscitation: A progress report. Resuscitation, 2000. 44: p. 219-30.
19) O’Neil, J., Deakin, CD., Do we hyperventilate cardiac arrest patients? Resuscitation, 2007. 73: p. 82-85.
20) Chandra, N.C., et al., Observations of hemodynamics during human cardiopulmonary resuscitation. Crit Care Med, 1990. 18: p. 929-34.
21) Aufderheide, T., Lurie, KG., Death by hyperventilation: A common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med, 2004. 32(No. 9 (Suppl.)): p. S345–S351.
22) Yannopoulos, D., Nadkarni, VM, McKnite, SH, Rao, A, Kruger, K, Metzger, J, Benditt, D, Lurie,KJ., Intrathoracic pressure regulator during continuous-chest-compression advanced cardiac resuscitation improves vital organ perfusion pressures in a porcine model of cardiac arrest. Circulation, 2005. 112: p. 803-81.
23) Guerci, A., Shi, AY, Levin, H, Tsitlik, J, Weisfeldt, ML, Chandra, N., Transmission of Intrathoracic Pressure to the Intracranial Space during Cardiopulmonary Resuscitation in Dogs. Circ Res, 1985. 56: p. 20-30.
24) Srinivasana, V., Nadkarnia, VA, Yannopoulosb, D, Marinoa, BS, Sigurdssonc, G, McKnitec, SH, Zookc, M, Bendittc, DG, Lurie, KG., Spontaneous gasping decreases intracranial pressure and improves cerebral perfusion in a pig model of ventricular fibrillation. Resuscitation, 2006. 69: p. 329-334
25) Yannopoulos, D., Nadkarni, VM, McKnite, SH, Rao, A, Kruger, K, Metzger, J, Benditt, D, Lurie,KJ., Intrathoracic pressure regulator during continuous-chest-compression advanced cardiac resuscitation improves vital organ perfusion pressures in a porcine model of cardiac arrest. Circulation, 2005. 112: p. 803-81.
26) Chandra, N.C., et al., Observations of hemodynamics during human cardiopulmonary resuscitation. Crit Care Med, 1990. 18: p. 929-34.
27) Lurie, K., Zielinski, T, Voelckel, W, et al., Augmentation of ventricular preload during treatment of cardiovascular collapse and cardiac arrest. Crit Care Med 2002. 30: p. (Suppl):S162-5.
28) Weil, M., Rackow, EC, Trevino, R, Grundler, W, Falk, JL, Griffel, MI., Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. N Engl J Med, 1986. 315: p. 153-156.
29) Sigurdsson, G., Yannopoulos, D, McKnite, S, et al., Lowering of intrathoracic pressure improves blood pressure and survival rates in a porcine model of hemorrhagic shock. Resuscitation, 2006. 68: p. 399-404.
30) Srinivasana, V., Nadkarnia, VA, Yannopoulosb, D, Marinoa, BS, Sigurdssonc, G, McKnitec, SH, Zookc, M, Bendittc, DG, Lurie, KG., Spontaneous gasping decreases intracranial pressure and improves cerebral perfusion in a pig model of ventricular fibrillation. Resuscitation, 2006. 69: p. 329-334.
31) Weil, M., Rackow, EC, Trevino, R, Grundler, W, Falk, JL, Griffel, MI., Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. N Engl J Med, 1986. 315: p. 153-156.
32) Leman P, Greene S, Whelan K, Legassick T., Simple lightweight disposable continuous positive airways pressure mask to effectively treat acute pulmonary oedema: Randomized controlled trial. Emergency Medicine Australasia, 2005. 17 p. 224 – 230.
33) Templier F, Dolveck F, Baer M, Chauvin M, Fletcher D., ‘Boussignac’ continuous positive airway pressure system: practical use in a prehospital medical care unit. Eur J Emerg Med, 2003. 10 P.87-93.
34) Malzer R, Zeiner A, Binder M, Domanovits H, Knappitsch G, Sterz F., Laggner AN. Hemodynamic effects of active compression-decompession after prolonged CPR Resuscitation, Volume 31, Issue 3, Pages 243-253
[I] While most exhalations are passive and do not require muscular assistance, we do sigh, cough and sometimes forcibly assist air out of our lungs and these instances of assisted exhalation are important to health.
[II] The German Otto Frank and Briton Ernest Starling.
[III] In the setting of resuscitation from cardiac arrest as opposed to CPS in cryonics Transports, the coronary perfusion pressure during CPR is the single most important predictor of successful defibrillation and thus of successful resuscitation.
[IV] It is important to note that this applies only in situations where the chest still possesses its basic structural integrity. In cases of rib fracture or ‘flail chest’ where the thorax has lost its rigidity, only ACD-CPR or the application of an integrated chest compressor and cuirass device would be effective in generating blood flow.