CPR: A pair of hands aren’t enough
CPR: A Pair of Hands Aren’t Enough: You Also Need a Heart and a Brain
“Anyone, anywhere, can now initiate cardiac resuscitation procedures. All that is needed are two hands.” [Kouwenhoven WB, Jude J, Knickerbocker G. Closed chest cardiac massage. JAMA 1960;173:1064–7.
Sudden Cardiac Arrest
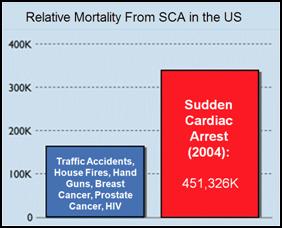
Figure 1: Mortality from sudden cardiac arrest (SCA) in 2004 as a result of myocardial infarction compared to death from other ‘high profile’ causes of mortality in the US.
Each year in the United States there are ~450,00 deaths from heart attack – myocardial infarction (MI)* with 310,000 of these deaths occurring before the patient reaches the hospital as a result of unsynchronized electrical activity in the heart, ventricular fibrillation, which stops the effective delivery of blood to the body. [2] This mode of sudden cardiac arrest[1, 2] (SCA) is also responsible for the majority of the 190,000 in-hospital deaths from MI, which typically occur within the first 24 hours following admission.[3] Especially tragic is that 50% of these deaths occur in persons ~60 years of age or less and thus lose at least a decade of active, productive life.[4] An estimated additional 20,000 incidents of SCA occur as a result of asphyxiation, drowning, electrocution, and genetic or developmental predisposition to lethal arrhythmias such as Wolf-Parkinson’s White Syndrome, congenital thickening of the interventricular septum, and idiopathic arrhythmic disease and other causes not related to non-atherosclerosis. This latter category of SCA typically occurs in individuals whose mean age is less than 35.[5, 6]
At this time the principal treatments for SCA consist of initiation of manual, ‘bystander’ cardiopulmonary resuscitation, so-called Basic Cardiac Life Support (BCLS or BLS), followed by ‘definitive’ treatment of the arrhythmia beginning with defibrillation and the application of Advanced Cardiac Life Support (ACLS or ALS).[7]
ACLS consists of the application of an algorithm of manual CPR, electrical defibrillation and pharmacologic therapy aimed at restoring a perfusing cardiac rhythm and adequate blood pressure and cardiac output to sustain life until definitive treatment of the underlying cause of the cardiac arrest can be achieved (e.g., coronary revascularization, implantation of an automatic defibrillator, or life-long anti-arrhythmic therapy).
What are the Odds?
![cpr_img2 Figure 2: Probability of survival as a function of time following cardiac arrest. [8]](https://www.biostasis.com/wp-content/uploads/2009/07/cpr_img2.jpg)
Figure 2: Probability of survival as a function of time following cardiac arrest. [8]
As is shown in Figure 3 below, the time to survival without neurological deficit following cardiac arrest in the absence of BCLS declines rapidly following a sigmoid curve with survival without neurological deficit being ~80-90% following 1 minute of arrest time, and less than 10% following 9 minutes of arrest.[8] Put another way, 50% of patients will experience significant morbidity or death following 4 minutes of circulatory arrest (Figure 2).
What is not shown in this graph is that the effect of immediate bystander CPR on survival is negligible in most studies [9,10] with the primary benefit being observed in patients who’s time from the initiation of BCLS to successful cardiac resuscitation was greater than 8 minutes.[11] There is evidence in the literature that morbidity is improved with prompt by-stander CPR [12] providing that EMS response is also rapid, although this remains controversial.[11,13] A corollary of this is that the overall survival rate following SCA, with or without serious neurological morbidity, ranges between 1% (New York City, NY) [14] to 17% (Seattle, WA).[15] The mean survival (defined as survival to discharge from the hospital) in the United States as a whole is generally agreed to be at best 15% [16] with ~70% of these patients experiencing lasting neurological morbidity (ranging from ‘mild’ cognitive impairment to total incapacitation in the Persistent Vegetative State (PVS).[17,18,19]
The primary cause of non-survival in patients experiencing SCA is failed cardiac or cerebral resuscitation. Arguably, it is failed cerebral resuscitation, since most underlying causes of refractory cardiac arrest could be treated by ‘bridging’ supportive technologies such as emergency femoral-femoral cardiopulmonary bypass (CPB) until myocardial revascularization and hemodynamic stabilization were achieved.[20] When emergency CPB is applied to patients who are candidates for good neurological outcome, the survival rate is increased.[21, 22, 23, 24] However, these technologies are not typically used on patients who are unsuccessfully resuscitated (restoration of adequate cardiac rhythm and perfusion) because of the justified perception that irreversible brain damage would have occurred during the prolonged period of cardiac arrest or CPR/ACLS.[21] Similarly, it is for this reason that most attempts to achieve cardiopulmonary resuscitation in hospitalized patients who are not hypothermic or intoxicated with sedative drug(s) are terminated after 15 minutes.[25, 26]
Within medicine it is widely understood that ‘CPR doesn’t really work’ and that if the return of spontaneous circulation (ROSC) is not achieved within ~ 5 minutes of cardiac arrest, the chances for survival are slim, and the chances for survival absent neurological impairment are slimmer still.[8] The principal reasons that conventional CPR is not effective are that it fails to supply an adequate amount of flow at an adequate pressure. Cardiac output (CO) is typically ~1/3rd of the at-rest requirement (~1.5 versus ~4.5 liters per minute), and mean arterial pressure (MAP) is typically 25 mm Hg to 45 mm Hg; well short of the 60 mmHg required to sustain cerebral viability.[27, 28]
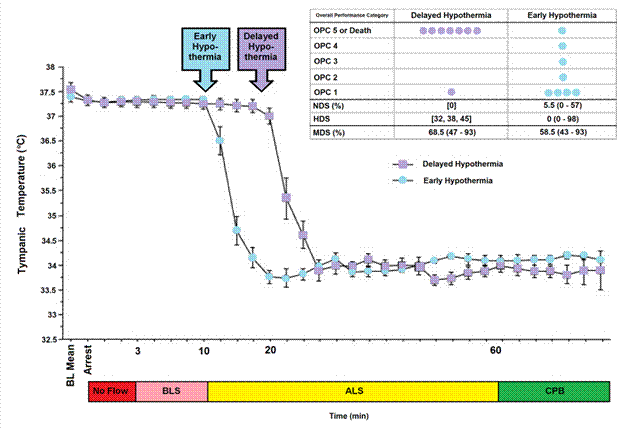
Figure 3: The impact of a delay of 10 min in inducing MHT is a dog model of cardiac arrest followed by 3 min of systemic ischemia, 7 minutes of mechanical CPR and 50 minutes of advanced life support. Hypothermia to 34oC was induced beginning at 10 min post arrest in the early hypothermia group˜ and at 20 min post arrest in the delayed hypothermia group ¢. In the early hypotherrmia group group, 5 of 7 surviving dogs were functionally normal (OPC 1 or 2), 1 had OPC 3, and 1 had OPC 4 (coma) at 96 hours of recovery. Histologically, 4 of 8 dogs in this group were normal (HDS 0), 1 had HDS 16, 1 had 22, and 1 had 98. The only surviving dog in the DH group was functionally normal at 96 hours (OPC 1, NDS 0) with an HDS of score of32 (mild injury) Due to early mortality only two other dogs in the delayed hypothermia group were evaluated histologically and their HDS scores 38 and 45, respectively. Dogs in this study were scored by overall performance categories (OPC; 1=normal, 2=moderate disability, 3=severe disability but conscious, 4=coma, and 5=death) Neurological function and neurological deficit scores (NDS; 0% to 10%=normal, 100%=brain death). [72],[73] Histological damage scores were obtained by neuropathological examination of 19 distcrete brain regions for severity and extent of ischemic neuronal changes, infarcts, and edema. A total brain histological damage score (HDS) >40 represented moderate damage, and HDS >100 represented severe damag.[74] Redrawn from Nozari, A., et al., Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation, 2006. 113(23): p. 2690-6.
The condition of the typical sudden cardiac arrest patient and the circumstances under which he experiences cardiac arrest are far from the ideal of a patient who is a candidate for emergency cardiopulmonary bypass in hospital. The typical SCA patient is middle aged or elderly, often suffering from one or more co-morbidities (diabetes, obesity, COPD, hypertension), and if subjected to prolonged CPR will invariably have impaired gas exchange due accumulation of fluid in both the parenchyma and the air-spaces of the lungs (pulmonary edema with alveolar flooding). This occurs because closed chest CPR quickly causes pulmonary edema.[29, 30] As previously noted, even when the SCA patient is a ‘good’ candidate for salvage; someone who is relatively young and free of co-morbidities, CPR will likely prove futile due to cerebral ischemia-reperfusion injury and the post-resuscitation syndrome.
Over the past 25 years a vast number of therapeutic interventions have shown great promise in animal models of regional and global cerebral ischemia in the laboratory.[31, 32, 33, 34] In the last 6 years alone, over 1000 experimental papers and over 400 clinical articles on pharmacological neuroprotection have been published.[35, 36] However, with one exception, none of these interventions has been successfully applied clinically despite many attempts. [37, 38, 39, 40, 41, 42, 43, 44] The sole exception to this frustrating debacle has been the introduction of moderate therapeutic hypothermia (MTH) as the standard of care for a select (and very small) minority of SCA patients.[45, 46, 47, 48, 49, 50, 51]
The question thus arises, how good is MTH? Is it, like CPR, just another ‘ritual therapy’ that works well in the laboratory but fails to deliver under the real world conditions of the in-field and clinical environments? And, of potentially great importance to cryonicists is the question of whether techniques being developed for clinical use in SCA have relevance or even merit direct application to the cryonics patient? The answer to those questions will be reviewed here in the near future.
References
1. American-Heart-Association, Heart Disease and Stroke Statistics – 2008 Update. . 2008, American Heart Association: Dallas, Texas.
2. de Vreede-Swagemakers, J., et al., Out-of-hospital cardiac arrest in the 1990’s: a population-based study in the Maastricht area on incidence, characteristics and survival. . J Am Coll Cardiol, 1997. 30: p. 1500-5.
3. American-Heart-Association-and-National-Research-Council, Standards for cardiopulmonary resuscitation (CPR) and emergency cardiac care (ECC). . JJ Amer Med Assoc, 1974. 227(suppl): p. 833-68.
4. Sakai, A., Sudden deaths among male employees: a six-year epidemiological survey. J Cardiol, 1990. 20: p. 957-61.
5. Safranek, D., Eisenberg, MS, Larsen, MP., The epidemiology of cardiac arrest in young adults. Ann Emerg Med, 1992. 21: p. 1102-6.
6. Viskin, S., Belhassen, B., Idiopathic ventricular fibrillation. American Heart Journal, 1990. 120: p. 661 – 671.
7. Skogvoll, E., et al., Out-of-hospital cardiopulmonary resuscitation: a population-based Norwegian study of incidence and survival. . Eur J Emerg Med, 1999. 6: p. 323-30.
8. Weale, F., The efficacy of cardiac massage. Lancet, 1960. 1: p. 990-96.
9. Eisenberg, M., Cardiac Arrest and Resuscitation: A tale of 29 cities. Ann of Emer Med, 1990. 19: p. 179-86.
10. Kentsch, M., et al., Early prediction of prognosis in out-of-hospital cardiac arrest. Intensive Care Med, 1990. 16: p. 378-83.
11. Troiano, P., et al., The effect of bystander CPR on neurologic outcome in survivors of prehospital cardiac arrests. Resuscitation, 1989. 17: p. 91-8.
12. Bossaert, L., Van Hoeyweghen, R., Bystander cardiopulmonary resuscitation (CPR) in out-of-hospital cardiac arrest. The Cerebral Resuscitation Study Group. . Resuscitation, 1989. 17(Suppl): p. S55-69; discussion S199-206.
13. Stueven, H., et al. , Bystander/first responder CPR: ten years experience in a paramedic system. Ann Emerg Med, 1986. 15: p. 707-10.
14. Lombardi, G., Gallagher, J, Gennis, P., Outcome of out-of-hospital cardiac arrest in New York City. The Pre- Hospital Arrest Survival Evaluation (PHASE) Study [see comments]. JAMA, 1994. 271: p. 678-83.
15. McCarthy, M., Looking after your neighbors Seattle-style. Lancet, 1998. 351: p. 732.
16. Hayward, M., Cardiopulmonary resuscitation: are practitioners being realistic? Br J Nurs, 1999. 8: p. 810-4.
17. Bengtsson, M., A psychiatric-psychosocial investigation of patients who had survived circulatory arrest. Acta Psychiat Scan, 1969. 45: p. 327.
18. Roewer, N., Kloss, T, Puschel, K., Long-term result and quality of life following preclinical cardiopulmonary resuscitation. Anasth Intensivther Notfallmed, 1985. 20: p. 244-50.
19. de Vos, R., Quality of life after cardiopulmonary resuscitation. Resuscitation, 1997. 35: p. 231-6.
20. Phillips, S., Resuscitation for cardiogenic shock with extracorporeal membrane oxygenation systems. Semin Thorac Cardiovasc Surg, 1994. 6: p. 131-5.
21. Younger, J., et al., Extracorporeal resuscitation of cardiac arrest [see comments]. Acad Emerg Med, 1999. 6: p. 700-7.
22. Matsuwaka, R., et al., Emergency percutaneous cardiopulmonary support for patients with cardiac arrest or severe cardiogenic shock. Nippon Kyobu Geka Gakkai Zasshi, 1996. 44: p. 2006-10.
23. Myerburg, R., Clinical, electrophysiologic, and hemodynamic profiles of patients resuscitated from pre-hospital cardiac arrest. . Amer J Med, 1980. 68: p. 568.
24. Safar, P., Abramson, NS, Angelos, M, et al., Emergency cardiopulmonary bypass for resuscitation from prolonged cardiac arrest. Am J Emerg Med, 1990. 8: p. 55-67.
25. Peterson, M., et al., Outcome after cardiopulmonary resuscitation in a medical intensive care unit. Chest, 1991. 100: p. 168-74.
26. Gener, J., et al., Immediate and 1-year survival after cardiopulmonary resuscitation at an intensive care unit. Med Clin (Barc), 1989. 93: p. 445-8.
27. Rubertsson, S., et al., Blood flow and perfusion pressure during open-chest versus closed-chest cardiopulmonary resuscitation in pigs. Am J Emerg Med, 1984. 23: p. 568-571.
28. Bircher, N., Safar, P., Open-chest CPR: An old method whose time has returned. Am J Emerg Med, 1984. 2: p. 568-71.
29. McDonald, J., Systolic and mean arterial pressures during manual and mechanical CPR in humans. Ann Emerg Med, 1982. 11: p. 292-5.
30. Ornato, J., et al., Measurement of ventilation during cardiopulmonary resuscitation. Crit Care Med., 1983. 1: p. 79-82.
31. Kim, H., et al., Amelioration of impaired cerebral metabolism after severe acidotic ischemia by tirilazad post-treatment in dogs. . Stroke, 1996. 27: p. 114-21.
32. Iwatsuki, N., et al., Hyperbaric oxygen combined with nicardipine administration accelerates neurologic recovery after cerebral ischemia in a canine model. . Crit Care Med, 1994. 22: p. 858-63.
33. Cervantes, M., Moralı´, G, Letechipı´a-Vallejo, G., Melatonin and ischemia reperfusion injury of the brain. J. Pineal Res, 2008. 45: p. 1-7.
34. Krep, H., Bernd W, Bottiger, BW, et al., Time course of circulatory and metabolic recovery of cat brain after cardiac arrest assessed by perfusion- and diffusion-weighted imaging and MR-spectroscopy. Resuscitation, 2003. 58: p. 337-348.
35. DeGraba, T., Pettigrew, C., Why do neroprotectivedrugs work in animals but not in humans? Neurologic Clinics, 2000. 18: p. 475-493.
36. Ginsberg, M., Adventures in the Pathophysiology of Brain Ischemia: Penumbra, Gene Expression, Neuroprotection. The 2002 Thomas Willis Lecture. Stroke, 2003. 34: p. 214-223.
37. Cheng, J., Al-Khoury, L, Zivin, JA., Neuroprotection for Ischemic Stroke: Two Decades of Success and Failure. NeuroRX, 2004. 1: p. 36-45.
38. Roine, R.O., et al., Nimodipine after resuscitation from out-of-hospital ventricular fibrillation. A placebo-controlled, double-blind, randomized trial. JAMA, 1990. 264(24): p. 3171-3177.
39. Longstreth, W.T., Jr., et al., Randomized clinical trial of magnesium, diazepam, or both after out-of-hospital cardiac arrest. Neurology, 2002. 59(4): p. 506-514.
40. Landau, W.M., et al., Randomized clinical trial of magnesium, diazepam, or both after out-of-hospital cardiac arrest. Neurology, 2003. 60(11): p. 1868-1869.
41. Halstrom, A., Rea, TD, et al., Manual chest compression vs use of an automated chest compression device during resuscitation following out-of-hospital cardiac arrest: a randomized trial. JAMA, 2006. 295: p. 2620-8.
42. Lafuente-Lafuente, C., Melero-Bascones, M., Active chest compression-decompression for cardiopulmonary resuscitation. Cochrane Database Syst Rev., 2004. (2):CD002751.
43. Aung, K., Htay, T., Vasopressin for cardiac arrest: a systematic review and meta-analysis. Arch Intern Med, 2005. 10: p. 17-24.
44. Callaham, M., Madsen, CD, Barton, CW, Saunders, CE, Pointer, J., A randomized clinical trial of high-dose epinephrine and norepinephrine vs standard-dose epinephrine in prehospital cardiac arrest. JAMA, 1992. 268: p. 2667-72.
45. Rincon, F., Mayer, SA., Therapeutic hypothermia for brain injury after cardiac arrest. . Semin Neurol 2006. 26: p. 387-395.
46. Bernard, S., Gray, TW, Buist, MD, et al., Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med, 2002. 346: p. 557-563.
47. Liu, L. and M.A. Yenari, Therapeutic hypothermia: neuroprotective mechanisms. Front Biosci, 2007. 12: p. 816-25.
48. The-Hypothermia-after-Cardiac-Arrest-Study-Group, Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med, 2002. 346: p. 549-556.
49. Nolan, J., Morley, PT, Vanden Hoek, TL, Hickey, RW., Therapeutic Hypothermia After Cardiac Arrest: An Advisory Statement by the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation. Circulation, 2003. 108: p. 118-121.
50. Arrich, J., Clinical application of mild therapeutic hypothermia after cardiac arrest. Crit Care Med, 2007. 35(4): p. 1041-7.
51. Sandroni, C., et al., In-hospital cardiac arrest: incidence, prognosis and possible measures to improve survival. Intensive Care Med, 2007. 33(2): p. 237-45.
* The author rejects the conventional designation of ‘sudden cardiac death’ because it is inaccurate; death is, by definition, the irreversible loss of life. Acute cardiac arrest is not death and the nomenclature used to describe it should reflect that fact.